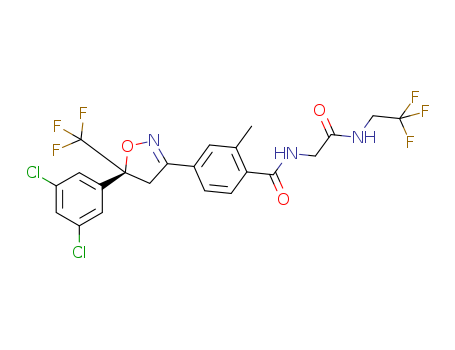
Product Details
Factory Supply fluralaner 864731-61-3 for Export at Lowest Price
- Molecular Formula:C22H17Cl2F6N3O3
- Molecular Weight:556.292
- PKA:12.50±0.46(Predicted)
- Density:1.51±0.1 g/cm3(Predicted)
Fluralaner(Cas 864731-61-3) Usage
|
Description |
Fluralaner, also known as AH252723, is a systemic insecticide and acaricide that is administered orally. |
|
Uses |
The US Food and Drug Administration (FDA) approved it under the trade name Bravecto for flea treatment in dogs in May 2014. The EU approved the drug in February 2014. Fluralaner inhibits γ-aminobutyric acid (GABA)-gated chloride channels (GABACls) and l-glutamate-gated chloride channels (GluCls). |
InChI:InChI=1S/C22H17Cl2F6N3O3/c1-11-4-12(2-3-16(11)19(35)31-9-18(34)32-10-21(25,26)27)17-8-20(36-33-17,22(28,29)30)13-5-14(23)7-15(24)6-13/h2-7H,8-10H2,1H3,(H,31,35)(H,32,34)
864731-61-3 Relevant articles
Synthetic method of fluralana
-
, (2022/04/15)
According to the method, 2-methyl-5-brom...
PROCESS FOR PREPARATION OF ISOXAZOLINE SUBSTITUTED BENZAMIDE COMPOUND
-
, (2022/03/14)
The invention relates to process for the...
PROCESS FOR PREPARING FLURALANER
-
Paragraph 0074-0082, (2021/06/26)
The invention relates to an improved pro...
Fluorine thramine intermediate and method for preparing flumbine by using same
-
Paragraph 0173-0175; 0182-0189, (2021/12/07)
The invention discloses a fluororesonide...
864731-61-3 Process route
-
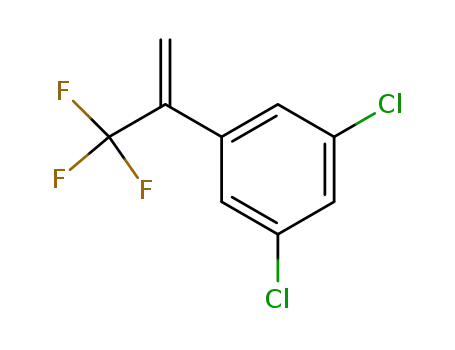
- 864725-22-4
1,3-dichloro-5-(3,3,3-trifluoroprop-1-en-2-yl)benzene

-
-
C13H13ClF3N3O3

-
- 864731-61-3
A1443
| Conditions | Yield |
|---|---|
|
With triethylamine; In N,N-dimethyl-formamide; at 20 ℃; for 14h;
|
92.1% |
|
With sodium hydrogencarbonate; In tetrahydrofuran; at 20 ℃;
|
91.8% |
|
for 3h;
|
83% |
-
![2-[[4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4H-isoxazol-3-yl]-2-methylbenzoyl]amino]acetic acid](/upload/2024/3/ac1a7177-fdbb-4e3e-a667-0a248cd3a1e2.png)
-
2-[[4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4H-isoxazol-3-yl]-2-methylbenzoyl]amino]acetic acid

-
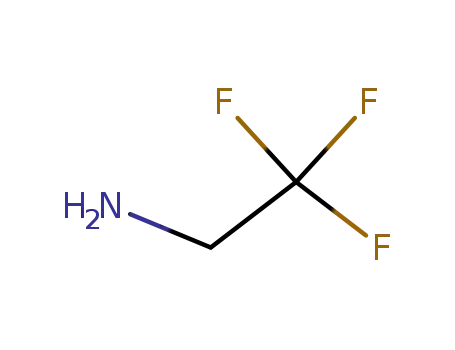
- 753-90-2
trifluoroethylamine

-
- 864731-61-3
A1443
| Conditions | Yield |
|---|---|
|
2-[[4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4H-isoxazol-3-yl]-2-methylbenzoyl]amino]acetic acid; trifluoroethylamine; With dmap; In dichloromethane; at 20 - 25 ℃;
With 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; In dichloromethane; Reagent/catalyst;
|
75.5% |
|
2-[[4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4H-isoxazol-3-yl]-2-methylbenzoyl]amino]acetic acid; With N-[3-(diethylamino)propyl]-N'-ethylcarbodiimide hydrochloride; In dichloromethane; at 20 ℃; for 0.25h;
trifluoroethylamine; With dmap; In dichloromethane; at 20 ℃; for 2h;
|
864731-61-3 Upstream products
-
753-90-2

trifluoroethylamine
-
201230-82-2
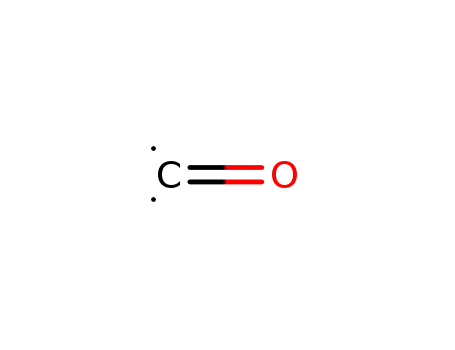
carbon monoxide
-
359821-38-8
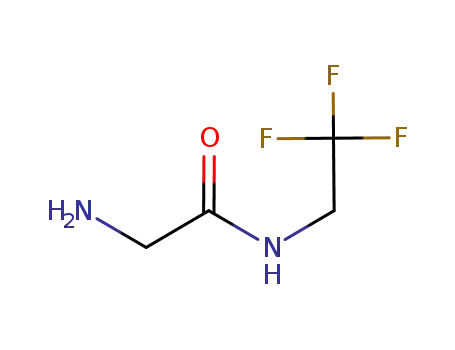
2-ammonia-N-(2,2,2-trifluoroethyl)acetamide
-
864725-62-2
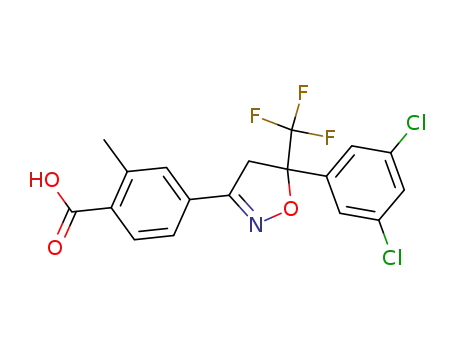
4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4,5-dihydro-1,2-oxazol-3-yl]-2-methylbenzoic acid
Relevant Products
-
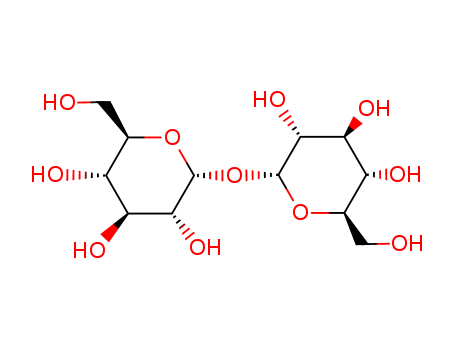
TREHALOSE
CAS:99-20-7
-
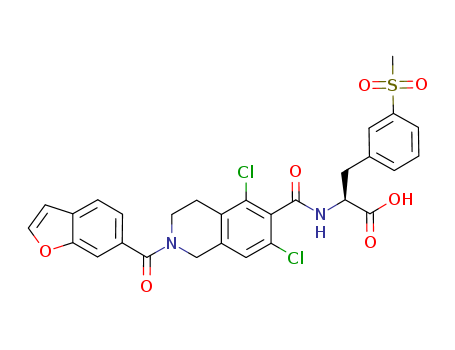
lifitegrast
CAS:1025967-78-5
-
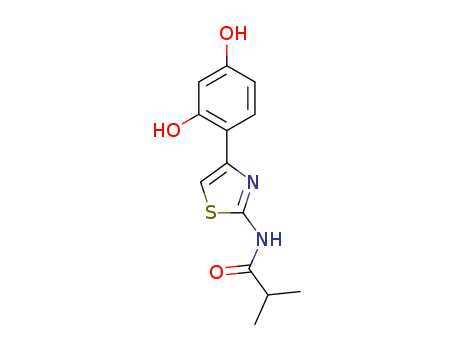
N-(4-(2,4-Dihydroxyphenyl)thiazol-2-yl)isobutyramide
CAS:1428450-95-6
-
Vidofludimus
CAS:717824-30-1