133242-30-5
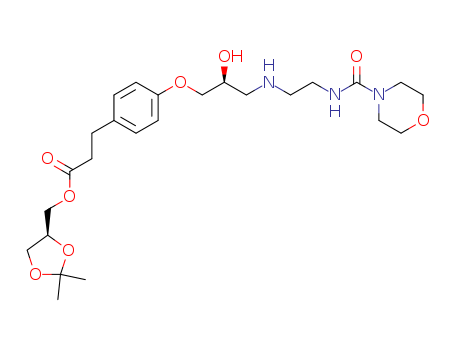
- Product Name:Landiolol
- Molecular Formula:C25H39N3O8
- Purity:99%
- Molecular Weight:509.6
Product Details
Buy Quality Landiolol 133242-30-5 of Top Purity 99% with Fast Shipping
- Molecular Formula:C25H39N3O8
- Molecular Weight:509.6
- Appearance/Colour:White crystalline powder
- Melting Point:125.4oC
- Boiling Point:727.513 °C at 760 mmHg
- Flash Point:393.786 °C
- PSA:127.82000
- Density:1.201 g/cm3
- LogP:1.40470
Landiolol(Cas 133242-30-5) Usage
|
Description |
Landiolol was launched as iv infusion for the treatment of tachyarrhythmia during surgery. This structurally related derivative of esmolol can be synthesized in 3 linear steps from 3-(4-hydroxyphenyl)propionic acid by successive esterification followed by alkylation of the phenol function with (2S)-glycidyltosylate and opening of the resulting epoxide by the appropriate amine. Landiolol is an ultra short acting PI-adrenergic blocker more cardioselective (βI/β2 = 255) than esmolol (βi/β2 = 32). It showed 6-8 times greater efficiency compared to esmolol in reducing isoproterenol-induced increase in heart rate and ventricular contraction in anesthetized dogs. In clinical trials, landiolol was effective against a variety of arrhythmias with efficacy seen in patients with atrial fibrillation, proxysmal supraventricular tachycardia, ventricular tachycardia and premature complexes. Landiolol produced a doserelated pharmacokinetic behavior, has a rapid onset of action (10 min.) and is rapidly hydrolyzed to inactive acidic metabolites by esterases after iv administration. This results in an ultra-short half-life (approx. 3 min.) and p-blocade, allowing rapid termination of the drug effect by termination of infusion if side effects occur. Hypertension was the most frequent adverse event and resolved in less than 30 min. after drug withdrawal. |
|
Uses |
Landiolol reduced heart rate significantly without reducing blood pressure, and stabilized hemodynamics. Landiolol is a short-acting beta-adrenergic receptor blocker that can be used to treat atrial tachyarrhythmias in patients with acute heart failure and cardiogenic shock . It's also used to control heart rate in emergencies, intensive care , and perioperative settings. Landiolol is highly selective for the β1 receptor and has a short half-life of 3–4.5 minutes. It's metabolized in the liver and plasma. |
InChI:InChI=1/C25H39N3O8.ClH/c1-25(2)35-17-22(36-25)21(14-23(30)32-3)18-4-6-20(7-5-18)34-16-19(29)15-26-8-9-27-24(31)28-10-12-33-13-11-28;/h4-7,19,21-22,26,29H,8-17H2,1-3H3,(H,27,31);1H/t19-,21?,22+;/m0./s1
133242-30-5 Relevant articles
Landiolol and Organ Failure in Patients With Septic Shock
Tony Whitehouse, MD1,2; Anower Hossain, PhD3; Gavin D. Perkins, MD1,3; Anthony C. Gordon, MD4; Julian Bion, MD1,5; Duncan Young, MD6; Danny McAuley, MD7,8; Mervyn Singer, MD9; Janet Lord, PhD2; Simon Gates, PhD10; Tonny Veenith, MD1,2; Niall S. MacCallum, PhD11; Joyce Yeung, MD1,3; Richard Innes, MD12; Ingeborg Welters, MD13; Nafisa Boota, MSc3; Emma Skilton, BSc3; Belinder Ghuman, BSc3; Maddy Hill, MPH3; Scott E. Regan, BA3; Dipesh Mistry, PhD3; Ranjit Lall, PhD3; for the STRESS-L Collaborators
, JAMA. 2023;330(17):1641-1652
To assess the efficacy and safety of landiolol in patients with tachycardia and established septic shock requiring prolonged (>24 hours) vasopressor support. Sixty-three patients were randomized to receive standard care and 63 to receive landiolol infusion.
Process for the enantioselective synthesis of landiolol
-
Paragraph 0019; 0062-0064, (2018/06/28)
PROBLEM TO BE SOLVED: To provide a proce...
Clinical role and efficacy of landiolol in the intensive care unit
Yuko Yoshida, Katsuyuki Terajima, Chiyo Sato, Shinji Akada, Yasuo Miyagi, Takashi Hongo, Shinhiro Takeda, Keiji Tanaka & Atsuhiro Sakamoto
, Journal of Anesthesia, Volume 22, pages 64–69, (2008)
The most common indication for landiolol in group S was the prevention of myocardial ischemia (50%), and in group IM, it was atrial fibrillation (45%). The median infusion rate of landiolol was 5 μg·kg−1·min−1 and the median infusion time was 2 days.
Process for the enantioselective synthesis of landiolol
-
Paragraph 0043; 0044, (2014/02/15)
A process for the preparation of Landiol...
133242-30-5 Upstream products
-
69630-16-6
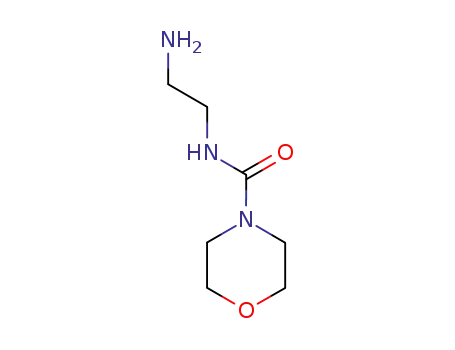
N-(2-aminoethyl)-4-morpholine carboxamide
-
144256-12-2
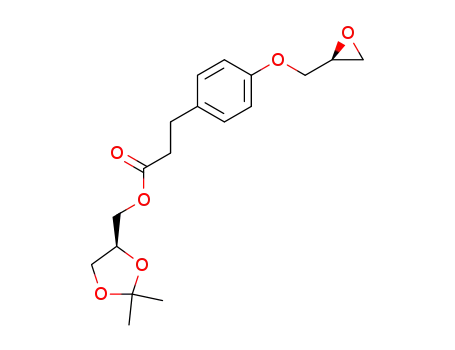
2,2-dimethyl-1,3-dioxolan-4S-ylmethyl 3-<4-<2(S),3-epoxypropoxy>phenyl>propionate
-
501-97-3
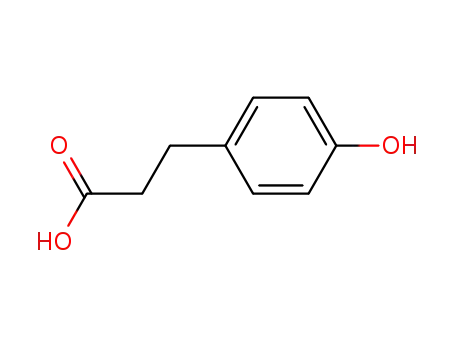
4-hydroxyphenylpropionic acid
-
93605-74-4
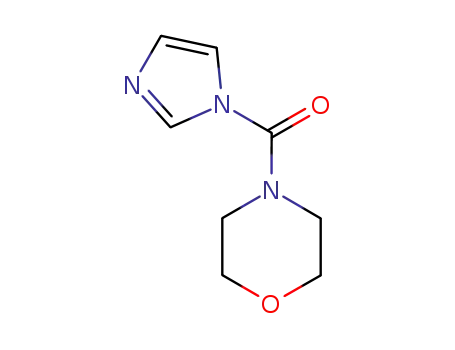
4-[(1H-imidazol-1-yl)carbonyl]morpholine
133242-30-5 Downstream products
-
144481-98-1
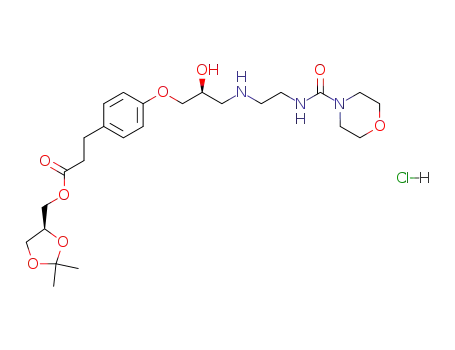
Landiolol hydrochloride
-
1253907-79-7
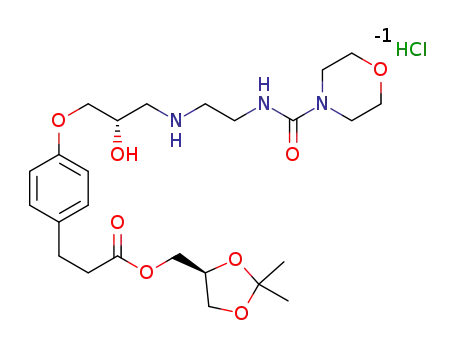
landiolol hydrochloride
Relevant Products
-
Sodium Citrate
CAS:76-46-0
-
all-trans-Retinol
CAS:68-26-8
-
chloramphenicol
CAS:56-75-7