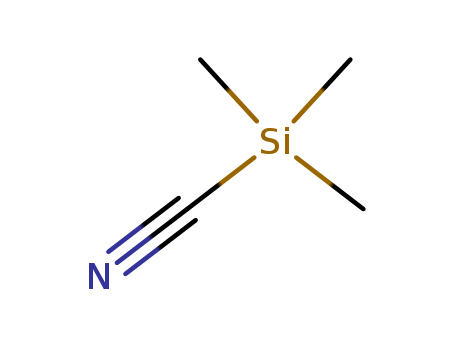
7677-24-9
- Product Name:Trimethylsilyl Cyanide
- Molecular Formula:C4H9NSi
- Purity:99%
- Molecular Weight:99.2077
Product Details
Buy High Grade Trimethylsilyl Cyanide 7677-24-9 of Best Quality with Best Price
- Molecular Formula:C4H9NSi
- Molecular Weight:99.2077
- Appearance/Colour:clear colorless to yellow liquid
- Vapor Pressure:16.6mmHg at 25°C
- Melting Point:8-11 °C(lit.)
- Refractive Index:n20/D 1.392(lit.)
- Boiling Point:118.499 °C at 760 mmHg
- Flash Point:1.111 °C
- PSA:23.79000
- Density:0.802 g/cm3
- LogP:1.38738
Trimethylsilyl cyanide(Cas 7677-24-9) Usage
|
Description |
Trimethylsilane cyanide is a colorless volatile liquid with almond odor, soluble in most organic solvents, such as dichloromethane, chloroform, etc.; it reacts violently with protic solvents such as water. This compound is used in organic synthesis as a replacement reagent for highly toxic HCN to introduce cyano groups into molecules. Trimethylsilyl cyanide (TMSCN) is a commercial reagent used as cyanide source for nucleophilic reactions. It is a clear colorless to yellow liquid, moisture sensitive, with a boiling point of 114–117 °C, which was first synthetized by the reaction of trimethylsilicon halides TMSX and AgCN in 1952.TMSCN is a versatile reagent that can be used in several different reactions, but it is generally used in nucleophilic additions to aldehydes, ketones and imines to form cyanohydrin silyl ethers (Scheme 1a) and α-aminonitriles in Strecker-type reactions (Scheme 1b).Reactions of Trimethylsilyl Cyanide |
|
Uses |
Cyanotrimethylsilane is a highly versatile reagent that reacts with a multitude of functional groups to yield an array of products and/or highly valuable synthetic intermediates.Aldehydes and ketones are readily transformed into the corresponding cyanohydrin trimethylsilyl ethers when treated with cyanotrimethylsilane in the presence of Lewis acids (eq 1), triethylamine,or solid bases such as CaF2 or hydroxyapatite. The products can be readily hydrolyzed to the corresponding cyanohydrins. The cyanosilylation of aromatic aldehydes can be achieved with high enantioselectivity in the presence of catalytic amounts of a modified Sharpless catalyst consisting of titanium tetraisopropoxide and L-(+)-diisopropyl tartrate (eq 2).Catalysis with chiral titanium reagents yields aliphatic and aromatic cyanohydrins in high chemical and optical yields (eq 3). Cyanohydrins can be subsequently transformed into a variety of useful synthetic intermediates (eq 4). |
InChI:InChI=1/C4H9NSi/c1-6(2,3)4-5/h1-3H3
7677-24-9 Relevant articles
An Improved Synthesis of Cyanotrimethylsilane
Reetz, M. T.,Chatziiosifidis, I.
, p. 330 (1982)
-
Free and Assosiated Trimethylsilyl Cation in Solution
Lambert, Joseph B.,McConnell, JoAnn A.,Schilf, Wojciech,Schulz, William J.
, p. 455 - 456 (1988)
The trimethylsilyl cation (Me3Si+) has b...
Alkoxycyanoborates: Metal salts and low-viscosity ionic liquids
Finze, Maik,Ignat'Ev, Nikolai V.,Reiss, Guido J.,Schopper, Nils,Sprenger, Jan A. P.,Zapf, Ludwig
, p. 14973 - 14987 (2021/09/04)
Syntheses of alkoxytricyanoborates and d...
Perfluoroalkyltricyanoborate and Perfluoroalkylcyanofluoroborate Anions: Building Blocks for Low-Viscosity Ionic Liquids
Landmann, Johannes,Sprenger, Jan A. P.,Hennig, Philipp T.,Bertermann, Rüdiger,Grüne, Matthias,Würthner, Frank,Ignat'ev, Nikolai V.,Finze, Maik
supporting information, p. 608 - 623 (2017/11/21)
The potassium perfluoroalkyltricyanobora...
Di- and tetrametallic hafnocene oxamidides prepared from CO-induced N 2 bond cleavage and thermal rearrangement to hafnocene cyanide derivatives
Semproni, Scott P.,Margulieux, Grant W.,Chirik, Paul J.
, p. 6278 - 6287,10 (2020/08/24)
Carbonylation of the hafnocene dinitroge...
IONIC COMPOUND, PROCESS FOR PRODUCING SAME, AND ION-CONDUCTIVE MATERIAL COMPRISING SAME
-
Page/Page column 44, (2011/06/23)
The present invention provides a method ...
7677-24-9 Process route
-
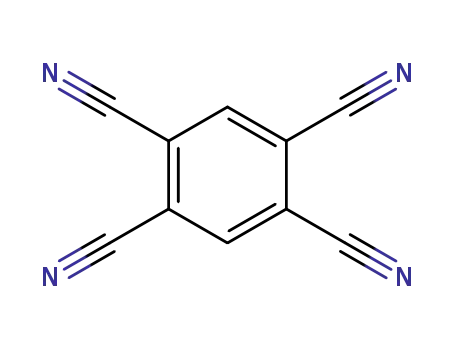
- 712-74-3
1,2,4,5-tetracyanobenzene

-
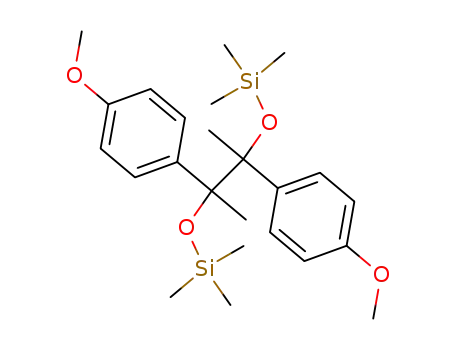
- 115352-07-3
2,3-bis(trimethylsiloxy)-2,3-bis(4'-methoxyphenyl)butane

-
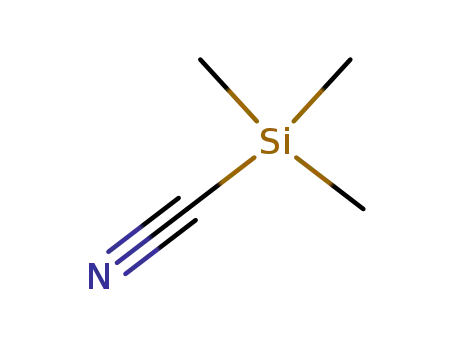
- 7677-24-9
trimethylsilyl cyanide

-
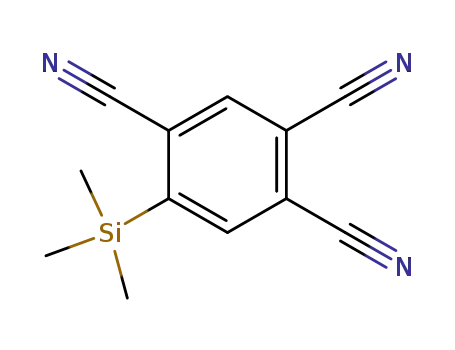
-
2,4,5-tricyano-trimethylsilylbenzene

-
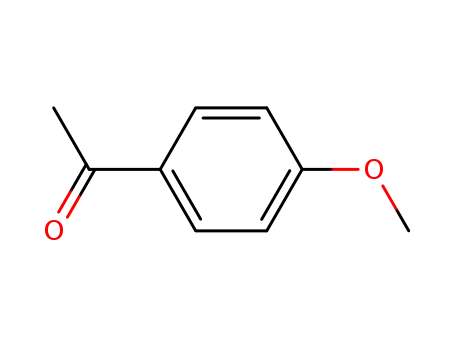
- 100-06-1
1-(4-methoxyphenyl)ethanone
| Conditions | Yield |
|---|---|
|
In dichloromethane; at 25 ℃; Product distribution; Quantum yield; Irradiation; various irradiation;
|
-

- 712-74-3
1,2,4,5-tetracyanobenzene

-
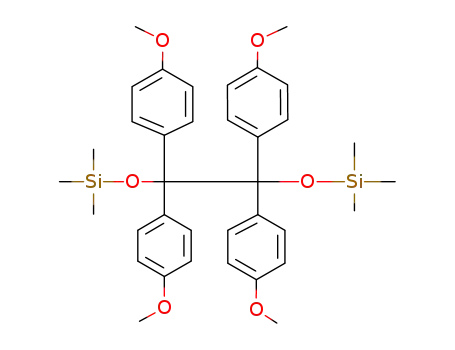
- 58405-33-7
1,1,2,2-tetrakis(4-methoxyphenyl)-1,2-(trimethylsiloxy)ethane

-
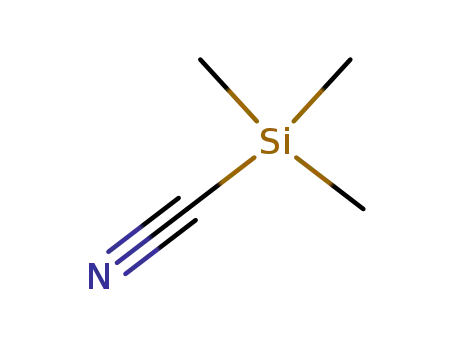
- 7677-24-9
trimethylsilyl cyanide

-
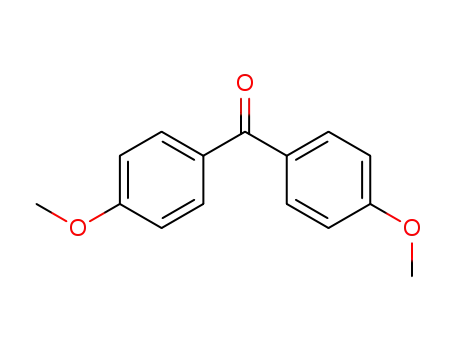
- 90-96-0
bis(p-methoxyphenyl)methanone

-

-
2,4,5-tricyano-trimethylsilylbenzene
| Conditions | Yield |
|---|---|
|
In dichloromethane; at 25 ℃; Product distribution; Quantum yield; Irradiation;
|
7677-24-9 Upstream products
-
75-77-4
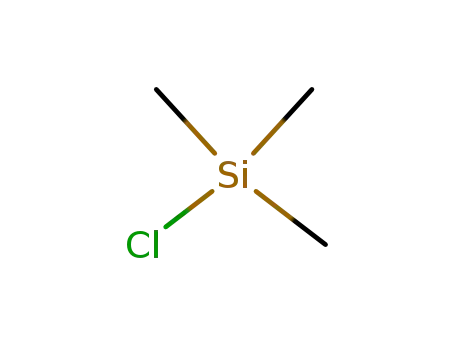
chloro-trimethyl-silane
-
74-90-8

hydrogen cyanide
-
506-64-9
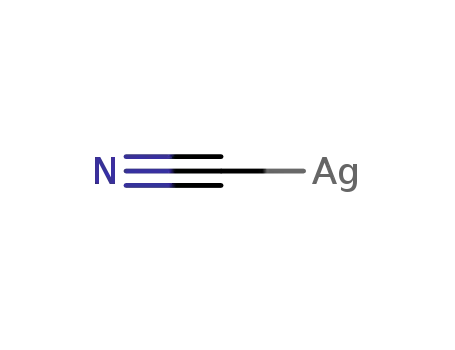
silver(I) cyanide
-
3385-94-2
hexamethyldisilathiane
7677-24-9 Downstream products
-
18678-64-3
triphenyl-silanecarbonitrile
-
72674-76-1
2-(4-methoxy-phenyl)-5-(4-methoxy-thiobenzoyl)-7-trimethylsilanylimino-[1,3,5]thiadiazepine-4,6-dione
-
52460-99-8
amino-diphenyl-acetonitrile
-
40326-25-8
2,2-diphenyl-2-(trimethylsiloxy)ethanenitrile
Relevant Products
-
Acesulfame
CAS:33665-90-6
-
Cyclohexanone cyanohydrin
CAS:931-97-5
-
2-nitrobenzaldehyde
CAS:552-89-6