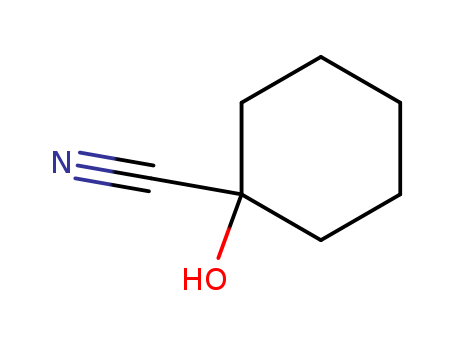
931-97-5
- Product Name:Cyclohexanone cyanohydrin
- Molecular Formula:C7H11 N O
- Purity:99%
- Molecular Weight:125.17
Product Details
Quality Factory Supply Best Quality Cyclohexanone cyanohydrin 931-97-5 with Efficient Delivery
- Molecular Formula:C7H11 N O
- Molecular Weight:125.17
- Appearance/Colour:Colourless solid. Typical sharp nitrile odor
- Vapor Pressure:0.0113mmHg at 25°C
- Melting Point:32-35ºC
- Refractive Index:1.4693
- Boiling Point:232.1 °C at 760 mmHg
- PKA:11.45±0.20(Predicted)
- Flash Point:112.1 °C
- PSA:44.02000
- Density:1.06 g/cm3
- LogP:1.20518
1-HYDROXY-1-CYCLOHEXANECARBONITRILE(Cas 931-97-5) Usage
|
Description |
Cyclohexanone cyanohydrin is a brown solid. |
| Uses | Cyclohexanone cyanohydrin was employed as substrate during high-throughput screening assay for hydroxynitrile lyase activity. It was used in preparation of 1-aminomethyl cyclohexanol via catalytic hydrogenation reaction. |
InChI:InChI=1/C7H11NO/c8-6-7(9)4-2-1-3-5-7/h9H,1-5H2
931-97-5 Relevant articles
CO2-Enabled Cyanohydrin Synthesis and Facile Iterative Homologation Reactions**
Juhl, Martin,Petersen, Allan R.,Lee, Ji-Woong
supporting information, p. 228 - 232 (2020/11/30)
Thermodynamic and kinetic control of a c...
Radical-mediated aerobic oxidation of substituted styrenes and stilbenes
Aman, Hasil,Chiu, Wei-Hua,Chuang, Gary Jing,Liu, Pin-Heng
, p. 20103 - 20106 (2021/12/02)
A 2,2-azobis(isobutyronitrile)-catalyzed...
Production process of high-purity and high-yield spirodiclofen
-
Paragraph 0046; 0051; 0067, (2019/03/08)
The invention relates to the technical f...
SPIRO-OXAZOLONES
-
Page/Page column 75, (2015/09/28)
The present invention provides spiro-oxa...
931-97-5 Process route
-
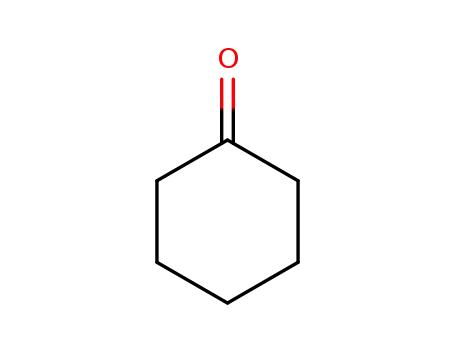
- 108-94-1,11119-77-0,9003-41-2,9075-99-4
cyclohexanone

-
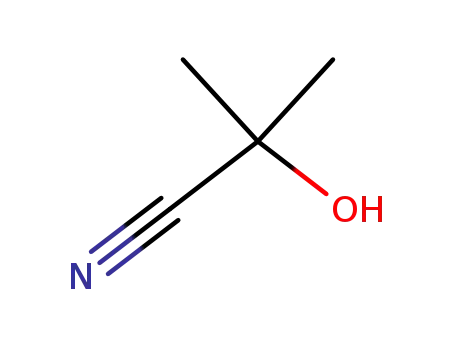
- 75-86-5
2-hydroxy-2-methylpropanenitrile

-
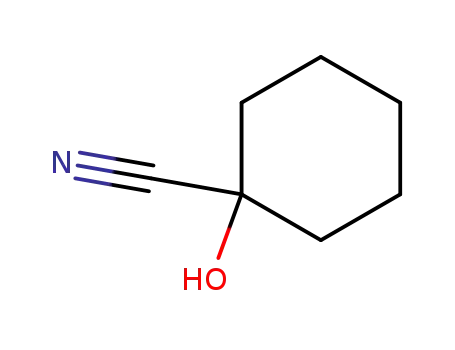
- 931-97-5
1-hydroxy-1-cyclohexanecarbonitrile
Conditions
| Conditions | Yield |
|---|---|
|
With Hevea brasiliensis (S)-hydroxynitrile lyase; pH=4.5; aq. buffer; Enzymatic reaction;
|
100% |
|
With titanium(IV) isopropylate; dl-3-(2-hydroxy-1-naphthylidene)-imino-ε-caprolactam (Nap-ACL); In dichloromethane; for 3h; Ambient temperature;
|
99% |
|
With ytterbium(III) isopropoxide; In tetrahydrofuran; for 0.5h; Ambient temperature;
|
94% |
|
With lanthanum(III) isopropoxide; In tetrahydrofuran; at 20 ℃;
|
81% |
|
With sodium hydroxide; In di-isopropyl ether; at 20 ℃; for 6h;
|
68% |
|
With potassium carbonate;
|
-

- 74-90-8
hydrogen cyanide

-

- 108-94-1,11119-77-0,9003-41-2,9075-99-4
cyclohexanone

-

- 931-97-5
1-hydroxy-1-cyclohexanecarbonitrile
Conditions
| Conditions | Yield |
|---|---|
|
in Gegenwart von Kaliumcarbonat,-hydroxyd oder -cyanid;
|
|
|
|
|
|
With basic ion exchanger;
|
|
|
With potassium hydroxide;
|
|
|
With citrate-phosphate buffer; Arabidopsis thaliana hydroxynitrile lyase; In di-isopropyl ether; at 10 ℃; for 3h; pH=5.0;
|
931-97-5 Upstream products
-
123-91-1
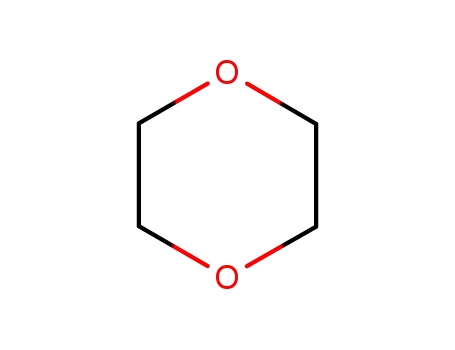
1,4-dioxane
-
74-90-8

hydrogen cyanide
-
108-94-1

cyclohexanone
-
64-17-5
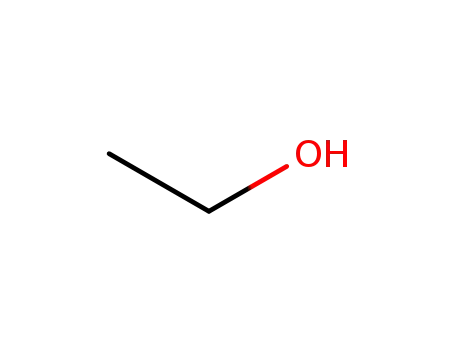
ethanol
931-97-5 Downstream products
-
1206-23-1
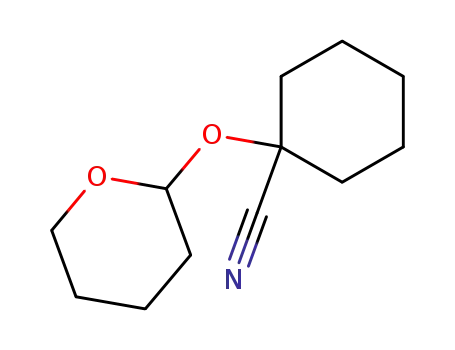
1-tetrahydropyran-2-yloxy-cyclohexanecarbonitrile
-
51939-79-8
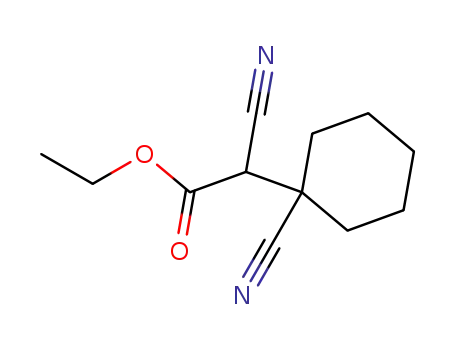
ethyl 2-cyano-2-(1-cyanocyclohexyl)acetate
-
74-90-8

hydrogen cyanide
-
108-94-1

cyclohexanone
Relevant Products
-
Sodium Citrate
CAS:76-46-0
-
Anthramycin
CAS:4803-27-4
-
Trimethylsilyl Cyanide
CAS:7677-24-9