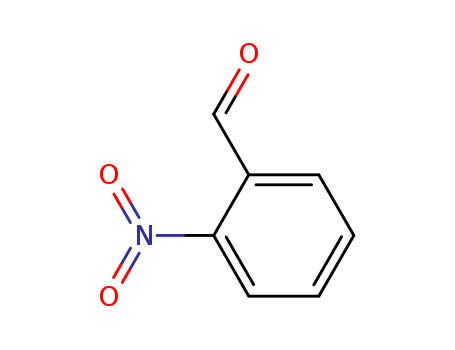
552-89-6
- Product Name:2-nitrobenzaldehyde
- Molecular Formula:C7H5NO3
- Purity:99%
- Molecular Weight:151.122
Product Details
Buy High Quality Wholesale 2-nitrobenzaldehyde 552-89-6 with Safe Shipping
- Molecular Formula:C7H5NO3
- Molecular Weight:151.122
- Appearance/Colour:Yellow crystalline powder or needles
- Vapor Pressure:0.0078mmHg at 25°C
- Melting Point:42-44 °C(lit.)
- Refractive Index:1.617
- Boiling Point:268.2 °C at 760 mmHg
- Flash Point:144 °C
- PSA:62.89000
- Density:1.338 g/cm3
- LogP:1.93050
2-Nitrobenzaldehyde(Cas 552-89-6) Usage
|
Description |
The 2-Nitrobenzyl group of 2-nitrobenzaldehyde is photolabile that can be cleaved when exposed to UV-light. 2-nitrobenzaldehydes are yellow or bright yellow needle-like crystals. It can volatilize with water vapor and has the fragrance of benzaldehyde. Soluble in ethanol, ether, benzene, slightly soluble in water, flash point>110℃, reacts violently with pyrrole. |
|
Uses |
2-Nitrobenzaldehyde is a benzaldhyde with a nitro group substituted in the ortho position. 2-Nitrobenzaldehyde is used in the preparation of dyes and colorants such as Indigo carmine. 2-Nitrobenzaldehyde gas been shown to be a useful photoremovable protecting group as well as in the preparation of more effective ones such as o-Nitrophenylethylene glycol. |
InChI:InChI=1/C7H5NO3/c9-5-6-3-1-2-4-7(6)8(10)11/h1-5H
552-89-6 Relevant articles
Synthesis of bimagnetic ionic liquid and application for selective aerobic oxidation of aromatic alcohols under mild conditions
Miao, Cheng-Xia,Wang, Jin-Quan,Yu, Bing,Cheng, Wei-Guo,Sun, Jian,Chanfreau, Sebastien,He, Liang-Nian,Zhang, Suo-Jiang
, p. 2697 - 2699 (2011)
The first bimagnetic ionic liquid based ...
Nitrolysis of 2,6,8,12-tetraacetyl-4,10-dibenzyl-2,4,6,8,10,12-hexaazatetracyclo[5.5.0.03,11.05,9]dodecane
Kalashnikov,Sysolyatin,Sakovich,Dubkov,Kulagina
, p. 531 - 536 (2017)
Nitrolysis of 2,6,8,12-tetraacetyl-4,10-...
Continuous flow nitration of benzaldehyde
Kulkarni, Amol A.,Kalyani, Vishwanath S.,Joshi, Ramesh A.,Joshi, Rohini R.
, p. 999 - 1002 (2009)
The nitration of benzaldehyde can be car...
Conversion of oximes into carbonyl compounds with thalium(III) nitrate supported onto HZSM-5 zeolite under microwave irradiation in solventless system
Heravi, Majid M.,Ghassemzadeh, Mitra
, p. 119 - 123 (2003)
Oximes are oxidatively deprotected by th...
Kinetics and Thermodynamics of Restricted Rotation of the Formyl Group in Nitrobenzaldehyde Anion Radicals
Branca, Mario,Gamba, Aldo,Barzaghi, Mario,Simonetta, Massimo
, p. 6506 - 6515 (1982)
The barrier to rotation about the carbon...
Oxidation of benzyl alcohols under mild heterogeneous conditions
Shirini,Zolfigol,Azadbar
, p. 1600 - 1602 (2001)
A combination of iodic acid with ammoniu...
Facile open air oxidation of benzylic alcohols in distilled water by in situ made copper(II) complexes
Ahmad, Jahir Uddin,R?is?nen, Minna T.,Kemell, Marianna,Heikkil?, Mikko J.,Leskel?, Markku,Repo, Timo
, p. 153 - 162 (2012)
A highly efficient, selective and green ...
Preparation method of o-nitrobenzaldehyde
-
, (2021/12/07)
The invention relates to the technical f...
Non-alkylator anti-glioblastoma agents induced cell cycle G2/M arrest and apoptosis: Design, in silico physicochemical and SAR studies of 2-aminoquinoline-3-carboxamides
Gu, Xiangyu,Liu, Jianwen,Ni, Xintong,Qi, Yingxue,Qian, Xuhong,Shao, Xusheng,Xu, Xiaoyong,Yuan, Pengtao
supporting information, (2021/09/22)
Malignant gliomas are the most common br...
The: In situ fabrication of ZIF-67 on titania-coated magnetic nanoparticles: A new platform for the immobilization of Pd(ii) with enhanced catalytic activity for organic transformations
Kaur, Manpreet,Paul, Satya,Sharma, Chandan,Sharma, Sukanya
, p. 20309 - 20322 (2021/11/22)
Considering the outstanding characterist...
Nitration of deactivated aromatic compounds via mechanochemical reaction
Wu, Jian-Wei,Zhang, Pu,Guo, Zhi-Xin
supporting information, (2021/05/05)
A variety of deactivated arenes were nit...
552-89-6 Process route
-
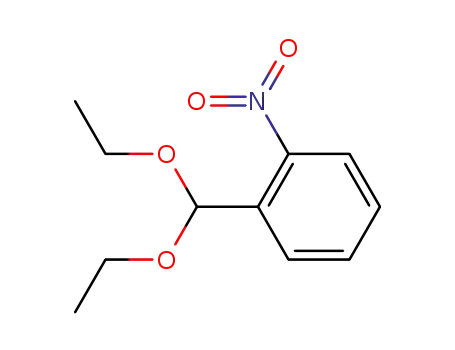
- 88356-11-0
o-nitrobenzaldehyde diethylacetal

-
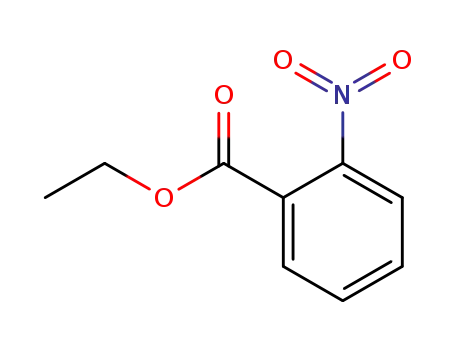
- 610-34-4
ethyl 2-nitrobenzoate

-
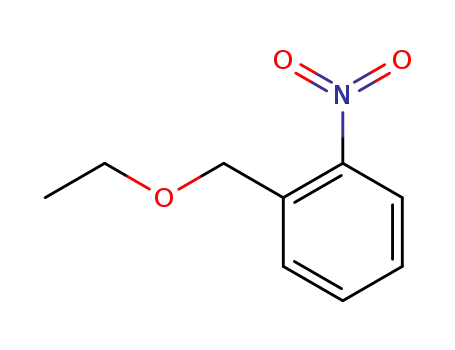
- 66424-93-9
2-ethoxymethyl-1-nitrobenzene

-
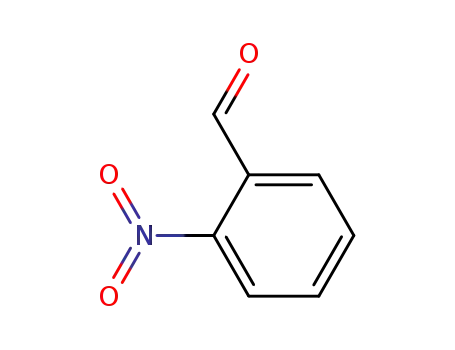
- 552-89-6
2-nitro-benzaldehyde
| Conditions | Yield |
|---|---|
|
aluminum oxide; In gas; at 350 ℃; Yield given;
|
|
|
aluminum oxide; In gas; at 200 ℃; Yield given;
|
-
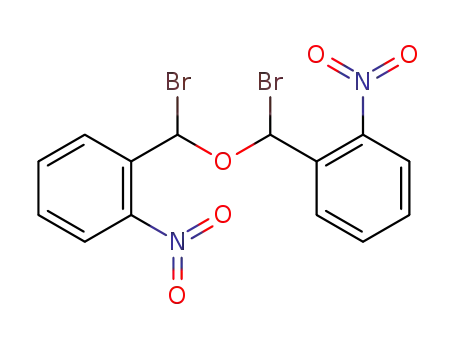
-
bis-(α-bromo-2-nitro-benzyl)-ether

-
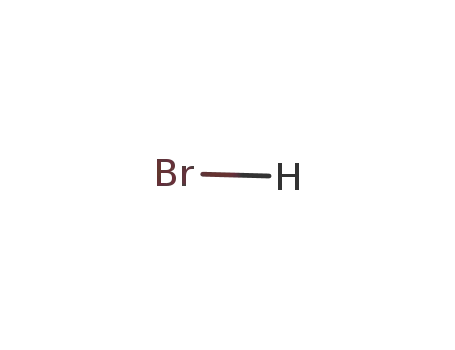
- 10035-10-6,12258-64-9
hydrogen bromide

-

- 552-89-6
2-nitro-benzaldehyde
| Conditions | Yield |
|---|---|
|
|
552-89-6 Upstream products
-
91-22-5
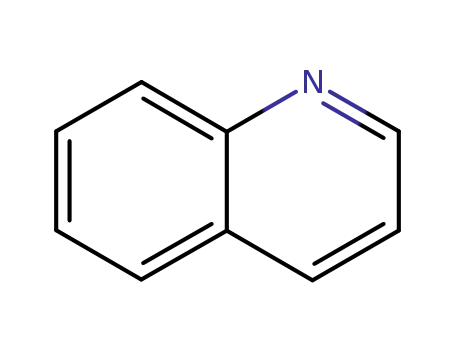
quinoline
-
99-65-0
meta-dinitrobenzene
-
612-25-9
2-Nitrobenzyl alcohol
-
497-19-8
sodium carbonate
552-89-6 Downstream products
-
577-59-3
2-acetylnitrobenzene
-
1969-72-8
(2-nitrophenyl)acetone
-
39830-70-1
(+/-)-2-(2-nitrophenyl)oxirane
-
124525-52-6
2-methyl-3t-(2-nitro-phenyl)-acrylic acid
Relevant Products
-
Sodium Citrate
CAS:76-46-0
-
Trimethylsilyl Cyanide
CAS:7677-24-9
-
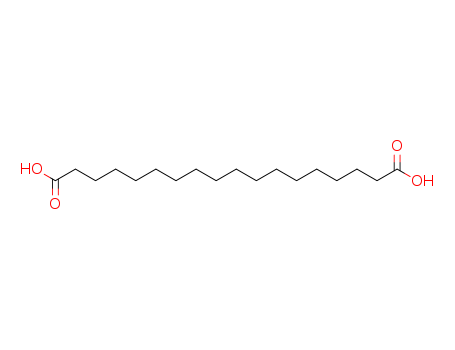
Octadecanedioic acid
CAS:871-70-5