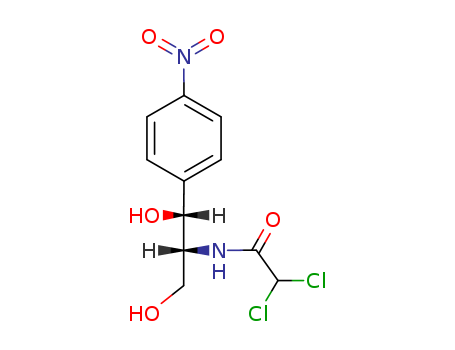
56-75-7
- Product Name:chloramphenicol
- Molecular Formula:C11H12Cl2N2O5
- Purity:99%
- Molecular Weight:323.133
Product Details
Chinese Manufacturer Supply Best Quality chloramphenicol, 56-75-7 Customized Supply Available
- Molecular Formula:C11H12Cl2N2O5
- Molecular Weight:323.133
- Appearance/Colour:White to grey-white crystalline powder
- Vapor Pressure:1.63E-17mmHg at 25°C
- Melting Point:148-150 °C(lit.)
- Refractive Index:20 ° (C=5, EtOH)
- Boiling Point:644.913 °C at 760 mmHg
- PKA:11.03±0.46(Predicted)
- Flash Point:343.831 °C
- PSA:115.38000
- Density:1.547 g/cm3
- LogP:1.82310
Chloramphenicol(Cas 56-75-7) Usage
|
Description |
Chloramphenicol, also known as chlornitromycin, is a broad-spectrum, bacteriostatic antibiotic derived from Streptomyces venezuelae. It is first isolated from cultures of Streptomyces venequelae in 1947 but now produced synthetically. The synthetic product is racemic, also called synthomycin. Syntomycin is a mixture of chloramphenicol L-isomer and d-isomer. Because of dextroisomer antibacterial effect, the effect of synthomycin is only half of the natural products. It has a relatively simple structure and was the first broad-spectrum antibiotic to be discovered. It is effective against several gram-positive and gram-negative bacteria and commonly used in researching protein synthesis and to select for chloramphenicol-resistant transformed cells or the bacterial CAT gene. Chloramphenicol is a semisynthetic, broad-spectrum antibiotic derived from Streptomyces venequelae with primarily bacteriostatic activity. Chloramphenicol diffuses through the bacterial cell wall and reversibly binds to the bacterial 50S ribosomal subunit. The binding interferes with peptidyl transferase activity, thereby prevents transfer of amino acids to the growing peptide chains and blocks peptide bond formation. As a result bacterial protein synthesis is blocked and impede bacterial cell proliferation. |
|
Uses |
Chloramphenicol is used for the treatment caused by typhoid bacillus, dysentery bacillus, Escherichia coli, bacillus, influenza and pneumococcal infections such as brucellosis. Chloramphenicol is used in the treatment of infections caused by bacteria. It works by killing bacteria or preventing their growth. Chloramphenicol is used to treat serious infections in different parts of the body. It is sometimes given with other antibiotics. However, chloramphenicol should not be used for colds, flu, other virus infections, sore throats or other minor infections, or to prevent infections. Chloramphenicol should only be used for serious infections in which other medicines do not work. This medicine may cause some serious side effects, including blood problems and eye problems. Symptoms of the blood problems include pale skin, sore throat and fever, unusual bleeding or bruising, and unusual tiredness or weakness. You and your doctor should talk about the good this medicine will do as well as the risks of taking it . Chloramphenicol is available only with your doctor's prescription. |
InChI:InChI=1/C11H12Cl2N2O5/c12-10(13)11(18)14-8(5-16)9(17)6-1-3-7(4-2-6)15(19)20/h1-4,8-10,16-17H,5H2,(H,14,18)/t8-,9?/m1/s1
56-75-7 Relevant articles
Catalytic Syn-Selective Nitroaldol Approach to Amphenicol Antibiotics: Evolution of a Unified Asymmetric Synthesis of (-)-Chloramphenicol, (-)-Azidamphenicol, (+)-Thiamphenicol, and (+)-Florfenicol
Chen, Fener,Cheng, Dang,Huang, Huashan,Jiang, Meifen,Liu, Minjie,Qu, Hongmin,Xia, Yingqi,Xiong, Tong,Zhang, Yan
, p. 11557 - 11570 (2021/09/02)
A unified strategy for an efficient and ...
Method for continuously preparing chloramphenicol by using micro-reaction system
-
Paragraph 0030-0044, (2021/08/19)
The invention belongs to the technical f...
Unified Strategy to Amphenicol Antibiotics: Asymmetric Synthesis of (-)-Chloramphenicol, (-)-Azidamphenicol, and (+)-Thiamphenicol and Its (+)-3-Floride
Liu, Jinxin,Li, Yaling,Ke, Miaolin,Liu, Minjie,Zhan, Pingping,Xiao, You-Cai,Chen, Fener
, p. 15360 - 15367 (2020/11/30)
The asymmetric synthesis of (-)-chloramp...
Oxidant controlled regio- and stereodivergent azidohydroxylation of alkenes via I2 catalysis
Prasad,Reddi,Sudalai
supporting information, p. 10276 - 10279 (2015/06/25)
A novel, I2 catalyzed regio- and stereod...
56-75-7 Process route
-
- 100381-03-1
(2RS,3SR)-2-(2,2-dichloro-acetylamino)-3-hydroxy-3-(4-nitro-phenyl)-propionic acid ethyl ester

-
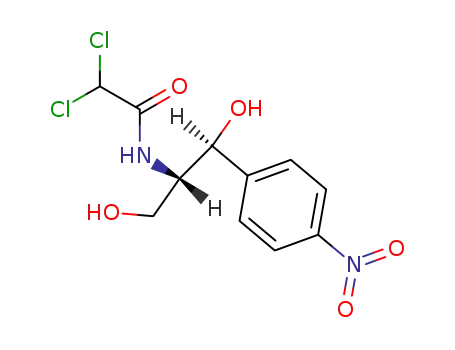
- 56-75-7,134-90-7,579-51-1,2787-09-9,3419-87-2,7384-89-6,7387-98-6,50762-99-7,126787-77-7,140694-65-1,85666-84-8
chlorampenicol
| Conditions | Yield |
|---|---|
|
With sodium tetrahydroborate; In methanol; at 0 ℃; for 0.5h;
|
79% |
-
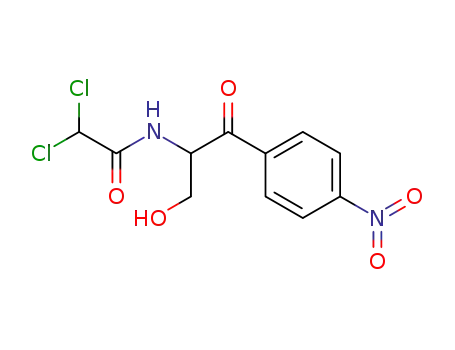
- 26367-75-9
chloramphenicol

-

- 56-75-7,134-90-7,579-51-1,2787-09-9,3419-87-2,7384-89-6,7387-98-6,50762-99-7,126787-77-7,140694-65-1,85666-84-8
chlorampenicol
| Conditions | Yield |
|---|---|
|
With aluminum isopropoxide; isopropyl alcohol;
|
|
|
With aluminium trichloride; aluminum isopropoxide; isopropyl alcohol;
|
56-75-7 Upstream products
-
79-36-7
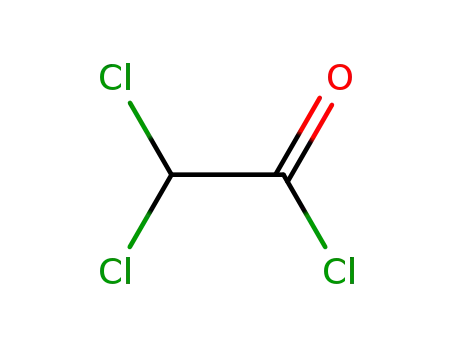
dichloroacethyl chloride
-
119-62-0
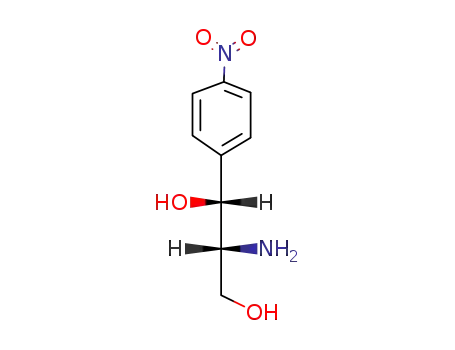
(1R,2R)-2-Amino-1-(4-nitrophenyl)-1,3-propanediol
-
302-17-0
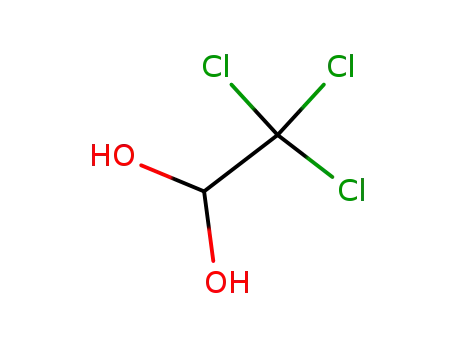
chloral hydrate
-
151-50-8
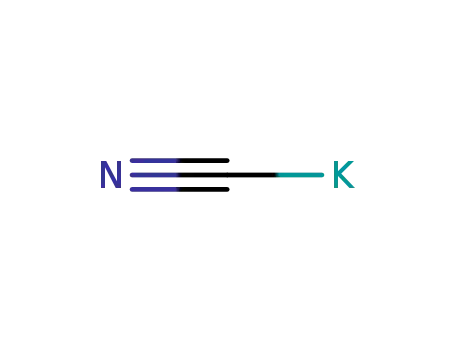
potassium cyanide
56-75-7 Downstream products
-
3544-94-3
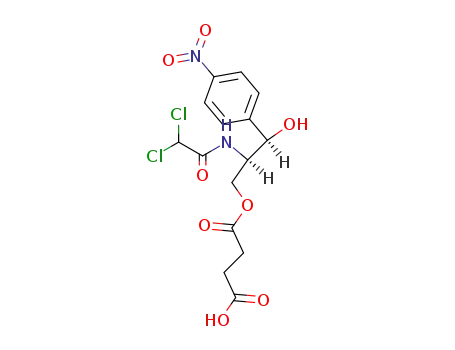
threo-(1R,2R)-1-(4-nitrophenyl)-2-(dichloroacetamido)-1,3-propanediol 3-succinate
-
33485-16-4
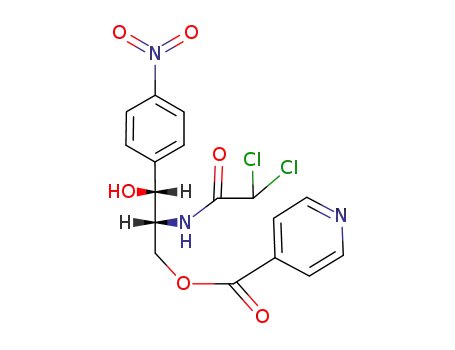
isonicotinic acid-[(2R,3R)-2-(2,2-dichloro-acetylamino)-3-hydroxy-3-(4-nitro-phenyl)-propyl ester]
-
130987-46-1
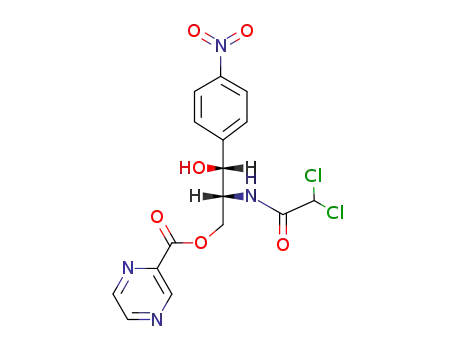
pyrazine-2-carboxylic acid-[(2R,3R)-2-(2,2-dichloro-acetylamino)-3-hydroxy-3-(4-nitro-phenyl)-propyl ester]
-
32307-90-7
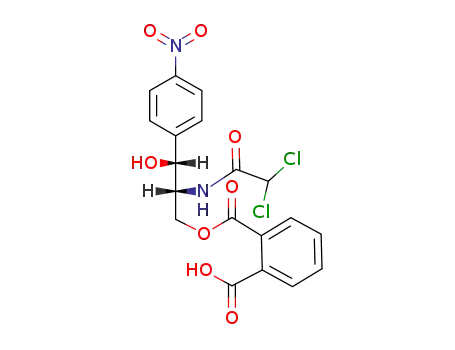
(1R,2R)-3-(2-carboxy-benzoyloxy)-2-(2,2-dichloro-acetylamino)-1-(4-nitro-phenyl)-propan-1-ol
Relevant Products
-
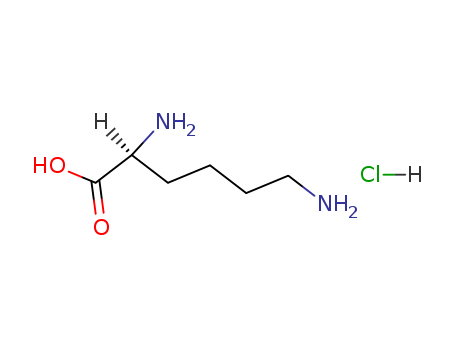
Lysine hcl
CAS:10098-89-2
-
Landiolol
CAS:133242-30-5
-
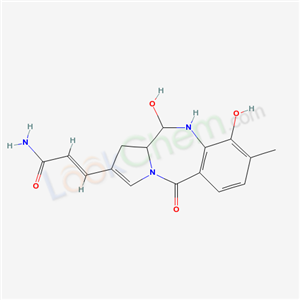
Anthramycin
CAS:4803-27-4