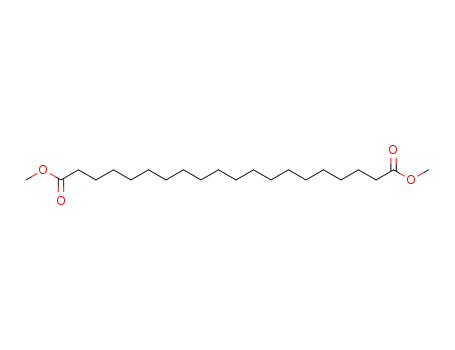
42235-38-1
- Product Name:dimethyl icosanedioate
- Molecular Formula:
- Purity:99%
- Molecular Weight:370.573
Product Details
Quality Manufacturer Supply High Purity dimethyl icosanedioate 42235-38-1 On Stock
- Molecular Formula:C22H42O4
- Molecular Weight:370.573
- Melting Point:66 °C
- Boiling Point:223 °C / 2mmHg
Dimethyl icosanedioate(Cas 42235-38-1) Usage
|
Description |
White to Almost white powder to crystal. |
|
Uses |
Dimethyl icosanedioate is commonly used as an additive in lubricating oils and lubricants to improve the lubrication performance and durability of products. It can also serve as a raw material for softeners in the textile, plastic, and rubber industries. Additionally, it can be used as an intermediate in chemical synthesis for synthesizing resins, dyes, pharmaceuticals, and other chemicals. |
InChI:InChI=1S/C22H42O4/c1-25-21(23)19-17-15-13-11-9-7-5-3-4-6-8-10-12-14-16-18-20-22(24)26-2/h3-20H2,1-2H3
42235-38-1 Relevant articles
A Facile and Efficient Method for the Synthesis of Labeled and Unlabeled Very Long Chain Polyunsaturated Fatty Acids
Hamberg, Mats
, p. 489 - 494 (2021/04/19)
Several methods are available for elonga...
Cysteine-Targeted Insecticides against A. gambiae Acetylcholinesterase Are Neither Selective nor Reversible Inhibitors
Gorecki, Lukas,Andrys, Rudolf,Schmidt, Monika,Kucera, Tomas,Psotka, Miroslav,Svobodova, Barbora,Hrabcova, Veronika,Hepnarova, Vendula,Bzonek, Petr,Jun, Daniel,Kuca, Kamil,Korabecny, Jan,Musilek, Kamil
, p. 65 - 71 (2019/12/25)
Acetylcholinesterase cysteine-targeted i...
Metathesis of renewable polyene feedstocks – Indirect evidences of the formation of catalytically active ruthenium allylidene species
Kovács, Ervin,Sághy, Péter,Turczel, Gábor,Tóth, Imre,Lendvay, Gy?rgy,Domján, Attila,Anastas, Paul T.,Tuba, Róbert
supporting information, p. 213 - 217 (2017/09/12)
Cross-metathesis (CM) of conjugated poly...
Synthesis of [3]rotaxanes that utilize the catalytic activity of a macrocyclic phenanthroline-Cu complex: Remarkable effect of the length of the axle precursor
Yamashita, Yoshiaki,Mutoh, Yuichiro,Yamasaki, Ryu,Kasama, Takeshi,Saito, Shinichi
supporting information, p. 2139 - 2145 (2015/02/19)
[3]Rotaxanes, which consist of one macro...
42235-38-1 Process route
-
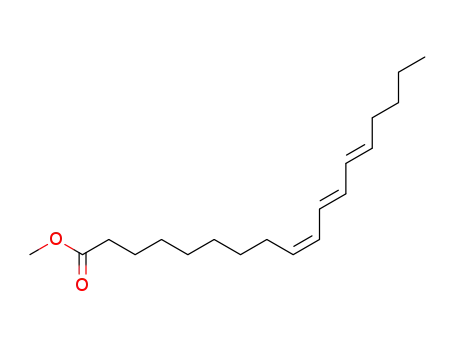
- 4175-47-7
methyl α-eleostearate

-
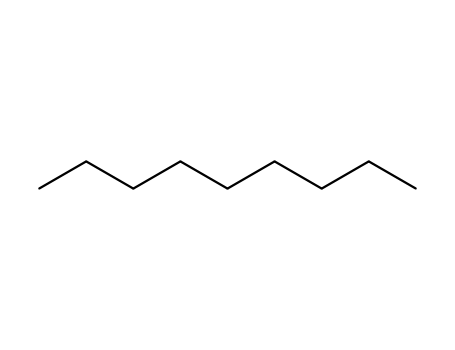
- 111-84-2
nonane

-
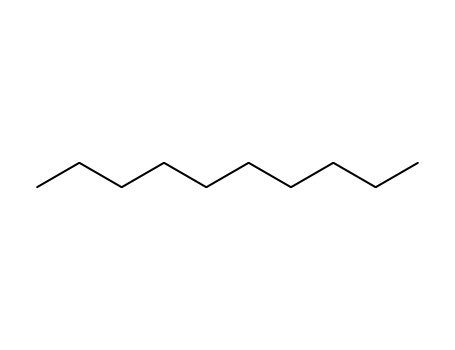
- 124-18-5
decane

-
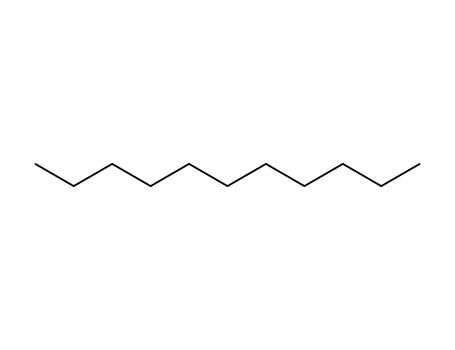
- 1120-21-4
n-Undecane

-
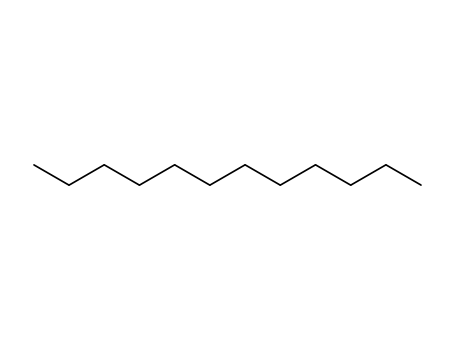
- 112-40-3
dodecane

-
- 629-50-5
Tridecane

-
- 629-59-4
tetradecane

-
- 544-76-3
Hexadecane

-
- 1731-88-0
methyl tridecanoate

-
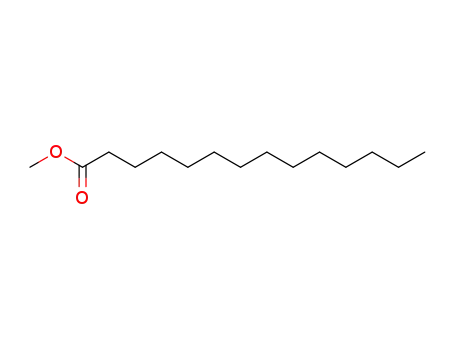
- 124-10-7
methyl myristoate

-
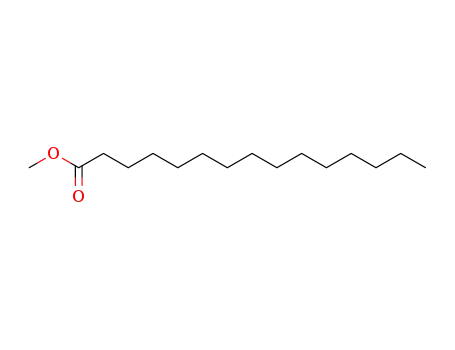
- 7132-64-1
pentadecanoic acid methyl ester

-
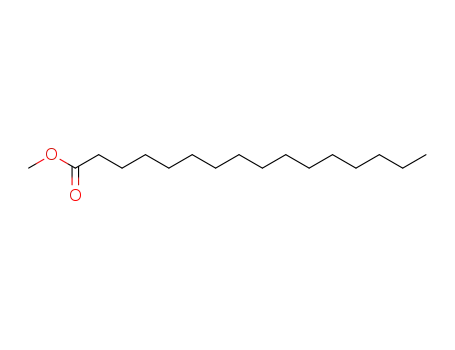
- 112-39-0
hexadecanoic acid methyl ester

-
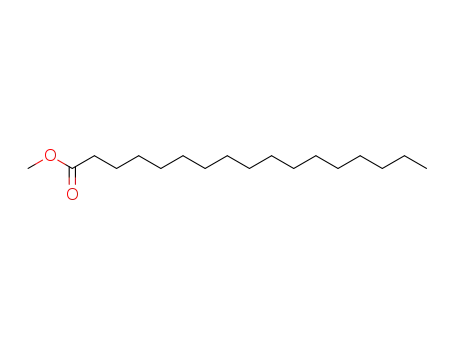
- 1731-92-6
methyl margarate

-
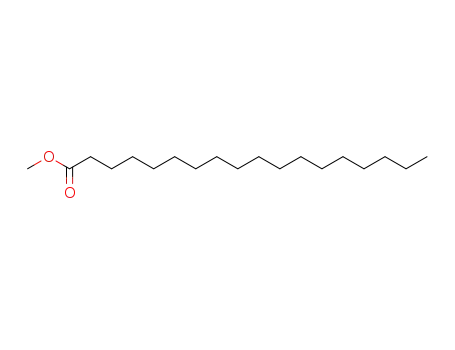
- 112-61-8
Methyl stearate

-
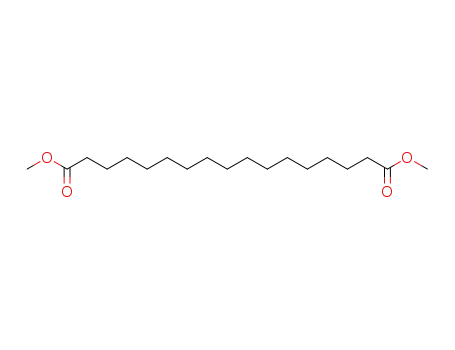
- 19102-92-2
dimethyl heptadecane-1,17-dioate

-
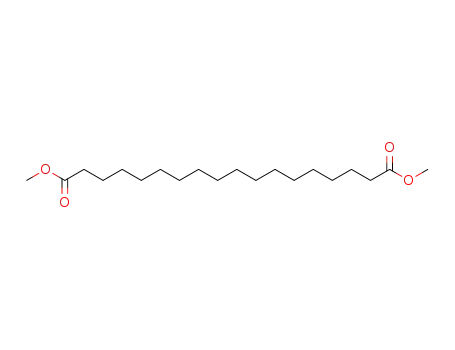
- 1472-93-1
dimethyl 1,18-octadecanedioate

-
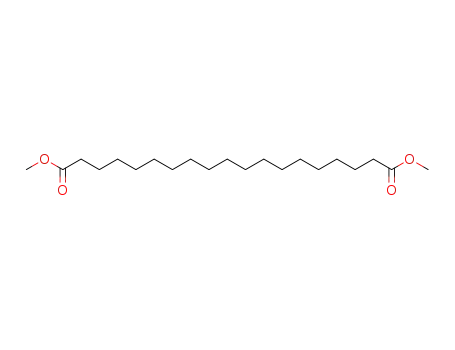
- 23130-41-8
dimethyl 1,19-nonadecandioate

-
- 42235-38-1
1,18-octadecanoic acid dimethyl ester

-
-
Methyl-2,6,10,14-tetramethylhexadecanoat
| Conditions | Yield |
|---|---|
|
methyl α-eleostearate; With tricyclohexylphosphine[1,3-bis(2,4,6-trimethylphenyl)-4,5-dihydroimidazol-2-ylidine][benzylidene]ruthenium(II) dichloride; In toluene; at 100 ℃; for 2h; Schlenk technique; Inert atmosphere;
With palladium on activated charcoal; hydrogen; In ethanol; toluene; at 20 ℃; Schlenk technique;
|
-

- 26825-94-5
10-bromodecanoic acid methyl ester

-
- 76971-26-1
(3Z)-8-bromooct-3-ene

-

- 42235-38-1
1,18-octadecanoic acid dimethyl ester

-
- 10411-39-9
methyl 15(Z)-octadecenoate

-
-
3,13-hexadecadiene
| Conditions | Yield |
|---|---|
|
With manganese; (1,2-dimethoxyethane)dichloronickel(II); 4,4',4-tri-tert-butyl-2,2':6',2-terpyridine; In N,N-dimethyl-formamide; at 40 ℃; for 4h;
|
0.22 mmol |
42235-38-1 Upstream products
-
67-56-1
methanol
-
2424-92-2
octadecane-1,18-dicarboxylic acid
-
53481-02-0
dimethyl 10-eicosenedioate
-
4175-47-7

methyl α-eleostearate
42235-38-1 Downstream products
-
1767-98-2
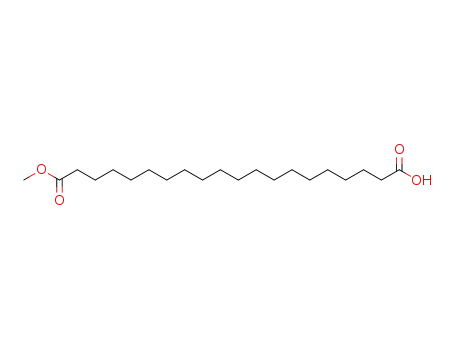
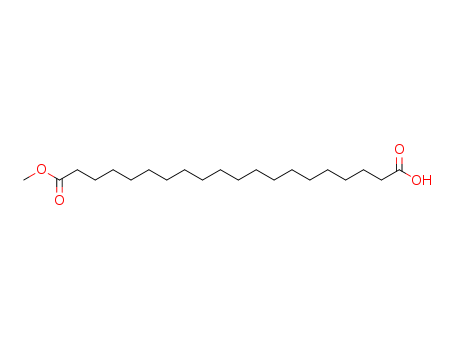
20-methoxy-20-oxoicosanoic acid
-
14296-16-3
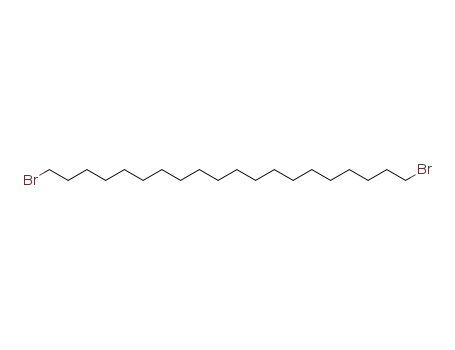
1,20-dibromoeicosane
-
838888-27-0
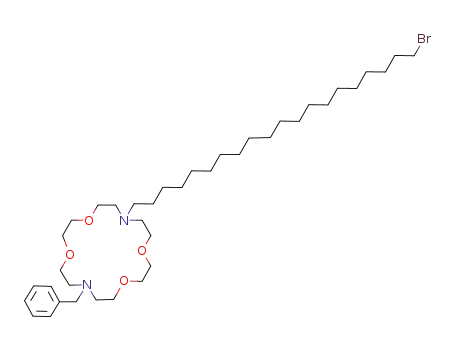
7-benzyl-16-(20-bromo-eicosyl)-1,4,10,13-tetraoxa-7,16-diaza-cyclooctadecane
-
6250-70-0
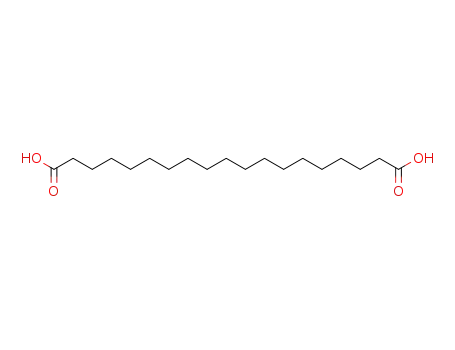
1,19-nonadecanedioic acid
Relevant Products
-
BIS-ETHYLHEXYLOXYPHENOL METHOXYPHENYL TRIAZINE
CAS:187393-00-6
-
Eicosan-1,20-disaeuremonomethylester
CAS:1767-98-2
-
10-hydroxycapric acid
CAS:1679-53-4