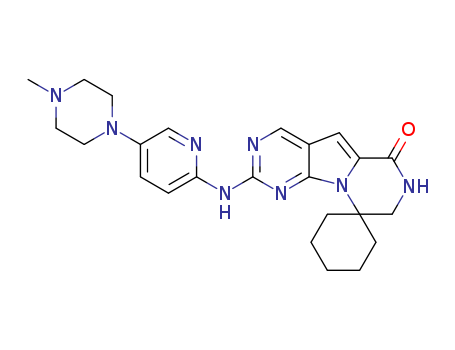
1374743-00-6
- Product Name:Trilaciclib
- Molecular Formula:
- Purity:99%
- Molecular Weight:446.555
Product Details
Quality Manufacturer Supply Top Purity Trilaciclib 1374743-00-6 at the Lowest Price
- Molecular Formula:C24H30N8O
- Molecular Weight:446.555
- Density:1.46±0.1 g/cm3(Predicted)
Trilaciclib(Cas 1374743-00-6) Usage
|
Description |
Trilaciclib is a Kinase Inhibitor. The mechanism of action of trilaciclib is as a Cyclin-dependent Kinase 4 Inhibitor, and Cyclin-dependent Kinase 6 Inhibitor, and Organic Cation Transporter 2 Inhibitor, and Multidrug and Toxin Extrusion Transporter 1 Inhibitor, and Multidrug and Toxin Extrusion Transporter 2 K Inhibitor. |
|
Uses |
Trilaciclib, or G1T28, is a CDK4 and CDK6 inhibitor, indicated to reduce the incidence of chemotherapy induced myelosuppression in patients before topotecan-containing or platinum and etoposide-containing chemotherapy for extensive stage small cell lung cancer. CDK4 and CDK6 inhibitors have been investigated since the mid 1990s for their use in tumorigenesis and chemotherapy. Trilaciclib was first described in the literature in 2016. Trilaciclib was granted FDA approval on 12 February 2021. |
InChI:InChI=1S/C24H30N8O/c1-30-9-11-31(12-10-30)18-5-6-20(25-15-18)28-23-26-14-17-13-19-22(33)27-16-24(7-3-2-4-8-24)32(19)21(17)29-23/h5-6,13-15H,2-4,7-12,16H2,1H3,(H,27,33)(H,25,26,28,29)
1374743-00-6 Relevant articles
SYNTHESIS OF N-(HETEROARYL)-PYRROLO[3,2-D]PYRIMIDIN-2-AMINES
-
Page/Page column 82; 83; 88; 89, (2018/02/28)
This invention is in the area of synthes...
COMBINATION THERAPY FOR THE TREATMENT OF CANCER
-
Page/Page column 89, (2018/07/31)
Compositions, combinations and methods c...
TREATMENT OF EGFR-DRIVEN CANCER WITH FEWER SIDE EFFECTS
-
Page/Page column 63-64, (2018/09/19)
The present invention provides methods f...
Transient Protection of Hematopoietic Stem and Progenitor Cells Against Ionizing Radiation
-
, (2014/09/29)
This invention is in the area of improve...
1374743-00-6 Process route
-
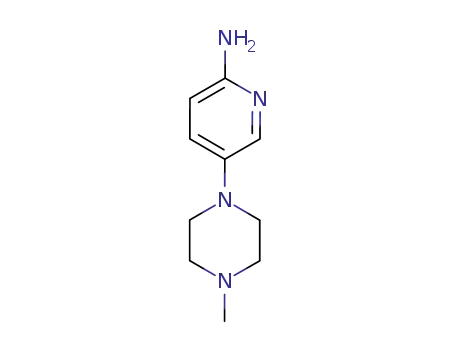
- 571189-49-6
5-(4-methyl-piperazin-1-yl)pyridin-2-ylamine

-
-
C15H18N4O3S

-
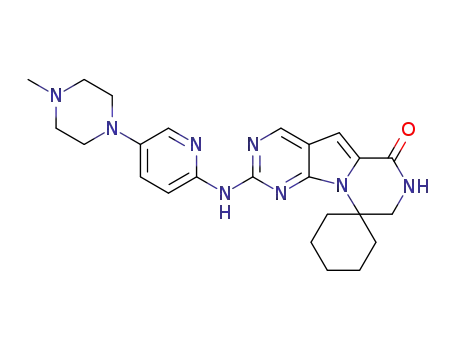
- 1374743-00-6
trilaciclib
| Conditions | Yield |
|---|---|
|
With lithium hexamethyldisilazane; In tetrahydrofuran; Inert atmosphere;
|
63.7% |
-
![tert-butyl [(1-aminocyclohexyl)methyl]carbamate](/upload/2024/3/eae996f2-ff7c-4af9-a0e9-fa81c2163ce9.png)
-
tert-butyl [(1-aminocyclohexyl)methyl]carbamate

-

- 1374743-00-6
trilaciclib
| Conditions | Yield |
|---|---|
|
Multi-step reaction with 10 steps
1: hydrogen; platinum on activated charcoal
2: hydrogenchloride / water
3: N-ethyl-N,N-diisopropylamine / N,N-dimethyl acetamide / 60 h
4: dmap / dichloromethane / 3 h / 20 °C
5: 1,8-diazabicyclo[5.4.0]undec-7-ene / tetrahydrofuran / 2 h
6: triethylamine / dichloromethane / 3 h / Inert atmosphere
7: triethylsilane; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; N,N-dimethyl-formamide / 14 h / 45 - 50 °C / Inert atmosphere
8: trifluoroacetic acid / dichloromethane / 20 °C
9: Oxone / acetonitrile; water
10: lithium hexamethyldisilazane / tetrahydrofuran / Inert atmosphere
With hydrogenchloride; triethylsilane; dmap; Oxone; tetrakis(triphenylphosphine) palladium(0); platinum on activated charcoal; hydrogen; 1,8-diazabicyclo[5.4.0]undec-7-ene; triethylamine; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid; lithium hexamethyldisilazane; In tetrahydrofuran; dichloromethane; N,N-dimethyl acetamide; water; N,N-dimethyl-formamide; acetonitrile;
|
|
|
Multi-step reaction with 11 steps
1: potassium carbonate / acetonitrile
2: 1,2-dichloro-ethane / Reflux
3: potassium carbonate / ethanol / 20 °C
4: N-ethyl-N,N-diisopropylamine / N,N-dimethyl acetamide / 60 h
5: dmap / dichloromethane / 3 h / 20 °C
6: 1,8-diazabicyclo[5.4.0]undec-7-ene / tetrahydrofuran / 2 h
7: triethylamine / dichloromethane / 3 h / Inert atmosphere
8: triethylsilane; tetrakis(triphenylphosphine) palladium(0) / tetrahydrofuran; N,N-dimethyl-formamide / 14 h / 45 - 50 °C / Inert atmosphere
9: trifluoroacetic acid / dichloromethane / 20 °C
10: Oxone / acetonitrile; water
11: lithium hexamethyldisilazane / tetrahydrofuran / Inert atmosphere
With triethylsilane; dmap; Oxone; tetrakis(triphenylphosphine) palladium(0); potassium carbonate; 1,8-diazabicyclo[5.4.0]undec-7-ene; triethylamine; N-ethyl-N,N-diisopropylamine; trifluoroacetic acid; lithium hexamethyldisilazane; In tetrahydrofuran; ethanol; dichloromethane; N,N-dimethyl acetamide; water; 1,2-dichloro-ethane; N,N-dimethyl-formamide; acetonitrile;
|
|
|
Multi-step reaction with 7 steps
1.1: hydrogen; platinum on activated charcoal
2.1: hydrogenchloride / water
3.1: N-ethyl-N,N-diisopropylamine / tert-butyl alcohol / 24 h / 80 - 85 °C
4.1: dmap / dichloromethane / 3 h / 20 °C
5.1: potassium tert-butylate / tetrahydrofuran / 2.17 h / 20 °C
5.2: 1 h
6.1: Oxone / acetonitrile; water
7.1: lithium hexamethyldisilazane / tetrahydrofuran / Inert atmosphere
With hydrogenchloride; dmap; Oxone; platinum on activated charcoal; potassium tert-butylate; hydrogen; N-ethyl-N,N-diisopropylamine; lithium hexamethyldisilazane; In tetrahydrofuran; dichloromethane; water; acetonitrile; tert-butyl alcohol;
|
|
|
Multi-step reaction with 7 steps
1.1: hydrogen; platinum on activated charcoal
2.1: hydrogenchloride / water
3.1: N-ethyl-N,N-diisopropylamine / tert-butyl alcohol
4.1: dmap / dichloromethane / 3 h / 20 °C
5.1: potassium tert-butylate / tetrahydrofuran / 2.17 h / 20 °C
5.2: 1 h
6.1: Oxone / acetonitrile; water
7.1: lithium hexamethyldisilazane / tetrahydrofuran / Inert atmosphere
With hydrogenchloride; dmap; Oxone; platinum on activated charcoal; potassium tert-butylate; hydrogen; N-ethyl-N,N-diisopropylamine; lithium hexamethyldisilazane; In tetrahydrofuran; dichloromethane; water; acetonitrile; tert-butyl alcohol;
|
|
|
Multi-step reaction with 8 steps
1.1: potassium carbonate / acetonitrile
2.1: 1,2-dichloro-ethane / Reflux
3.1: potassium carbonate / ethanol / 20 °C
4.1: N-ethyl-N,N-diisopropylamine / tert-butyl alcohol / 24 h / 80 - 85 °C
5.1: dmap / dichloromethane / 3 h / 20 °C
6.1: potassium tert-butylate / tetrahydrofuran / 2.17 h / 20 °C
6.2: 1 h
7.1: Oxone / acetonitrile; water
8.1: lithium hexamethyldisilazane / tetrahydrofuran / Inert atmosphere
With dmap; Oxone; potassium tert-butylate; potassium carbonate; N-ethyl-N,N-diisopropylamine; lithium hexamethyldisilazane; In tetrahydrofuran; ethanol; dichloromethane; water; 1,2-dichloro-ethane; acetonitrile; tert-butyl alcohol;
|
|
|
Multi-step reaction with 8 steps
1.1: potassium carbonate / acetonitrile
2.1: 1,2-dichloro-ethane / Reflux
3.1: potassium carbonate / ethanol / 20 °C
4.1: N-ethyl-N,N-diisopropylamine / tert-butyl alcohol
5.1: dmap / dichloromethane / 3 h / 20 °C
6.1: potassium tert-butylate / tetrahydrofuran / 2.17 h / 20 °C
6.2: 1 h
7.1: Oxone / acetonitrile; water
8.1: lithium hexamethyldisilazane / tetrahydrofuran / Inert atmosphere
With dmap; Oxone; potassium tert-butylate; potassium carbonate; N-ethyl-N,N-diisopropylamine; lithium hexamethyldisilazane; In tetrahydrofuran; ethanol; dichloromethane; water; 1,2-dichloro-ethane; acetonitrile; tert-butyl alcohol;
|
1374743-00-6 Upstream products
-
571189-49-6

5-(4-methyl-piperazin-1-yl)pyridin-2-ylamine
-
1374635-90-1
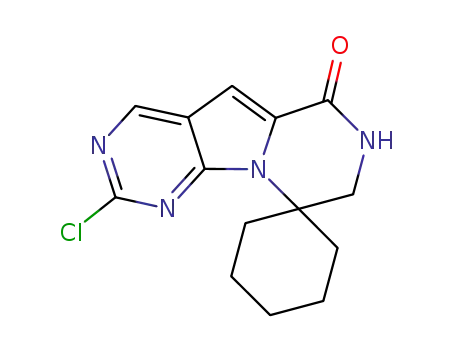
2’-chloro-7’,8’-dihydro-6’H-spiro[cyclohexane-1,9’-pyrazino[1,2:1,5]pyrrolo[2,3-d]pyrimidin]-6’-one
-
1374635-87-6
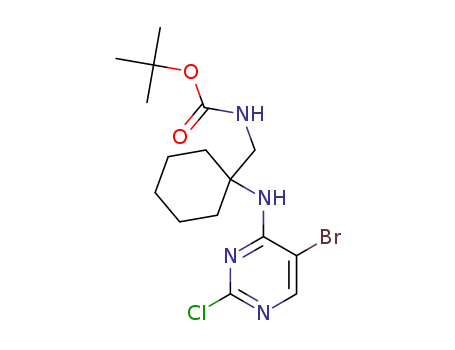
tert-butyl N-[[1-[(5-bromo-2-chloro-pyrimidin-4-yl)amino]cyclohexyl]methyl]carbamate
-
1374635-88-7
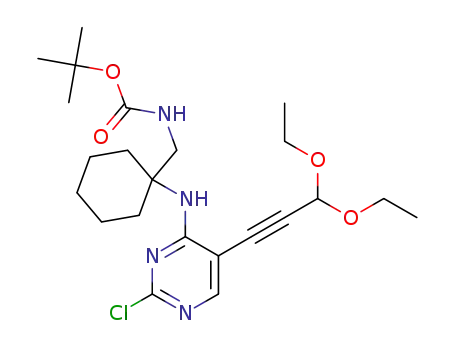
tert-butyl ((1-((2-chloro-5-(3,3-diethoxyprop-1-yn-1-yl)pyrimidin-4-yl)amino)cyclohexyl)methyl)carbamate
Relevant Products
-
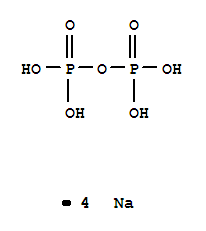
Tetrasodium pyrophosphate
CAS:7722-88-5
-
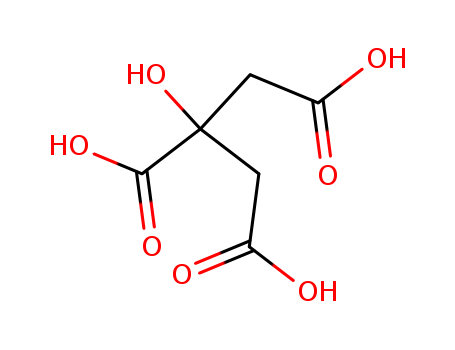
Citric acid
CAS:77-92-9
-
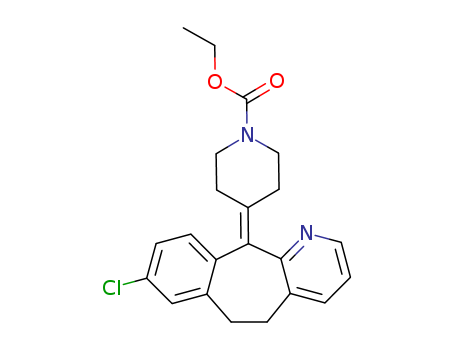
Loratadine
CAS:79794-75-5
-
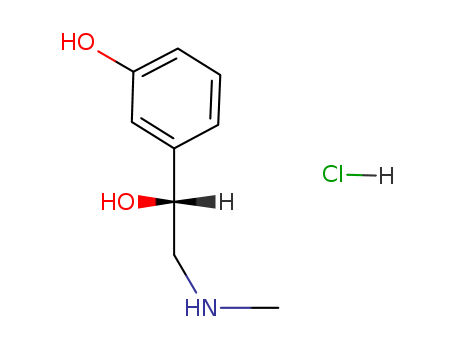
phenylephrine hydrochloride
CAS:61-76-7