Product Details
Factory Supply Top Purity MCP 7758-23-8 with Safe Delivery
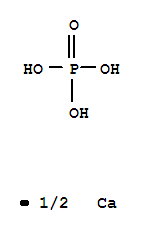
- Molecular Formula:Ca.(H2PO4)2
- Molecular Weight:234.05
- Appearance/Colour:white crystalline powder
- Boiling Point:158 °C at 760 mmHg
- PSA:180.80000
- Density:2.22(16/4 °C)
- LogP:-0.98080
Calcium dihydrogen orthophosphate(Cas 7758-23-8) Usage
|
Description |
Calcium dihydrogen orthophosphate, also known as monocalcium phosphate, is a chemical compound with the formula Ca(H2PO4)2. It is commonly found as a monohydrate which means it has one molecule of water (H2O) as part of its structure and is denoted as Ca(H2PO4)2.H2O. This chemical is often used as a leavening agent in the food industry, specifically in the production of baking powder and bread to promote rising. It is also commonly utilized in fertilizers and feed supplements in the agriculture industry due to its high nutrient content. Moreover, it is considered safe and non-toxic though excessive consumption can lead to health issues due to calcium and phosphorus imbalances in the body. |
|
Uses |
MCP is an inorganic compound with far-reaching applications across various domains of science and industry. It′s employed extensively in creating fertilizers and food additives, and contributes to the manufacturing process of ceramics. The production of cement and concrete also involves the use of this compound. Calcium biphosphate is integral to several biological processes, such as bone mineralization and energy metabolism. In the realm of dentistry, it′s used for crafting dental composites, sealants, and other materials. Furthermore, it′s a vital element in the production of animal feed and pet food. In biological context, it′s a crucial part of bone mineralization, contributing to the formation of hydroxyapatite, a key mineral component of bone. It′s involved in energy metabolism, providing a source of energy for cells. Moreover, it plays a role in pH regulation, being a source of bicarbonate ions that help maintain optimal pH levels. |
InChI:InChI=1/Ca.H3O4P.H2O/c;1-5(2,3)4;/h;(H3,1,2,3,4);1H2/q+2;;/p-1
Relevant Products
-
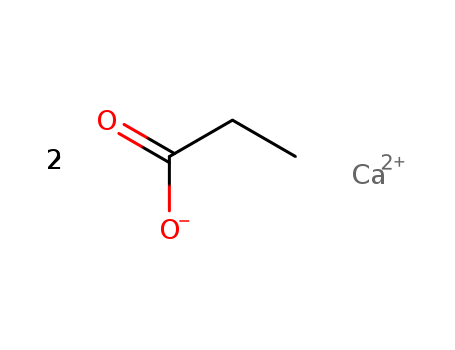
Calcium Propionate
CAS:4075-81-4
-
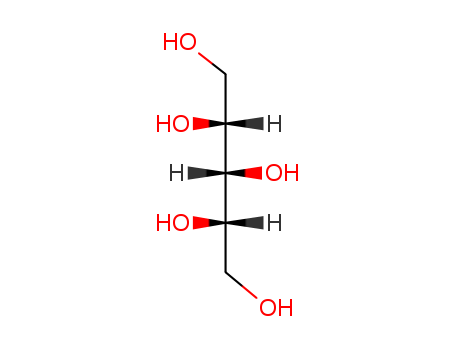
Xylitol
CAS:87-99-0
-
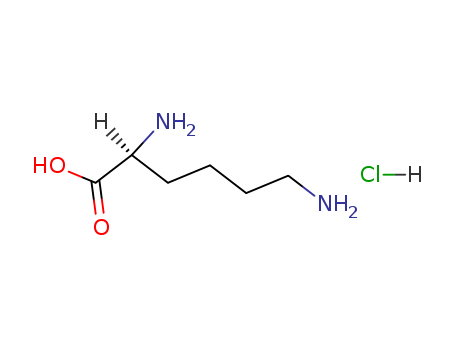
Lysine hcl
CAS:10098-89-2