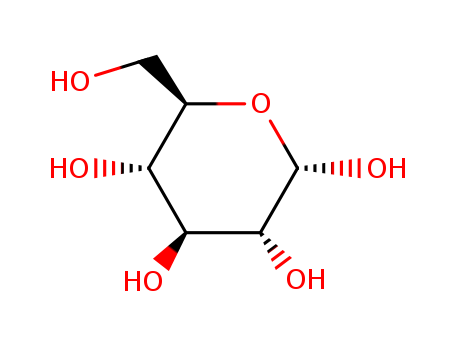
Product Details
Buy High Grade Glucose 492-62-6 with Reliable Quality On Stock
- Molecular Formula:C6H12O6
- Molecular Weight:180.158
- Appearance/Colour:white, odorless, fine crystalline powder odurless
- Melting Point:153-156 °C(lit.)
- Refractive Index:n20/D 1.362
- Boiling Point:410.797 °C at 760 mmHg
- PKA:12.12±0.70(Predicted)
- Flash Point:202.243 °C
- PSA:110.38000
- Density:1.732 g/cm3
- LogP:-3.22140
DEXTROSE(Cas 492-62-6) Usage
| Description |
White, odorless, fine crystalline powder |
|
Uses |
α-D-Glucose is used:As a reducing agent in the preparation superparamagnetic ferrous oxide (Fe3O4) nanoparticles and silver nanocrystals.As an additive for the formation of isoporous polystyrene-block-poly(4-vinylpyridine) (PS-b-P4VP) diblock copolymer membranes.For glycosylation of cell-penetrating poly(disulfide)s (CPDs) with improved solubility to achieve multifunctional cellular uptake.As a precursor in the synthesis of metal/carbon nanohybrids under hydrothermal conditions. |
InChI:InChI=1/C6H12O6/c7-1-2-3(8)4(9)5(10)6(11)12-2/h2-11H,1H2/t2-,3-,4+,5-,6?/m1/s1/i1+0,2+0,3+0,4+0,5+0,6+0
492-62-6 Relevant articles
Two new triterpenoid glycosides from Curculigo orchioides
Zuo, Ai-Xue,Shen, Yong,Jiang, Zhi-Yong,Zhang, Xue-Mei,Zhou, Jun,Lue, Jun,Chen, Ji-Jun
, p. 407 - 412 (2012)
Two new cycloartane triterpenoid glycosi...
Microbial production of neryl-α-D-glucopyranoside from nerol by Agrobacterium sp. M-12 reflects glucosyl transfer activity
Takahashi, Kazuki,Terauchi, Issei,Ono, Marie,Satoh, Hiroshi,Ueda, Makoto
, p. 2205 - 2211 (2018)
Terpene alcohol is widely used in perfum...
Aryl sulfonic acid catalyzed hydrolysis of cellulose in water
Amarasekara, Ananda S.,Wiredu, Bernard
, p. 259 - 262 (2012)
Catalytic activities of eight alkyl/aryl...
Immobilized cellulase on Fe3O4 nanoparticles as a magnetically recoverable biocatalyst for the decomposition of corncob
Zhang, Qikun,Kang, Junqing,Yang, Bing,Zhao, Leizhen,Hou, Zhaosheng,Tang, Bo
, p. 389 - 397 (2016)
A magnetically recoverable biocatalyst w...
Flavonoid glucuronides and a chromone from the aquatic macrophyte Stratiotes aloides
Conrad, Juergen,Foerster-Fromme, Bernhard,Constantin, Mihaela-Anca,Ondrus, Vladimir,Mika, Sabine,Mert-Balci, Fadime,Klaiber, Iris,Pfannstiel, Jens,Moeller, Wolfgang,Roesner, Harald,Foerster-Fromme, Karin,Beifuss, Uwe
, p. 835 - 840 (2009)
The first phytochemical analysis of the ...
Hydrolysis of α- and β-D-glucosyl fluoride by individual glucosidases: new evidence for separately controlled "plastic" and "conserved" phases in glycosylase catalysis
Matsui, Hirokazu,Tanaka, Yoshimasa,Brewer, Curtis F.,Blanchard, John S.,Hehre, Edward J.
, p. 45 - 56 (1993)
α-Glucosidases from sugar beet seed and ...
Structural elements responsible for the glucosidic linkage-selectivity of a glycoside hydrolase family 13 exo-glucosidase
Saburi, Wataru,Rachi-Otsuka, Hiroaki,Hondoh, Hironori,Okuyama, Masayuki,Mori, Haruhide,Kimura, Atsuo
, p. 865 - 869 (2015)
Abstract Glycoside hydrolase family 13 c...
Gluconic acid from biomass fast pyrolysis oils: Specialty chemicals from the thermochemical conversion of biomass
Santhanaraj, Daniel,Rover, Marjorie R.,Resasco, Daniel E.,Brown, Robert C.,Crossley, Steven
, p. 3132 - 3137 (2014)
Fast pyrolysis of biomass to produce a b...
Acremonoside, a phenolic glucoside from the sea fan-derived fungus Acremonium polychromum PSU-F125
Khamthong, Nanthaphong,Rukachaisirikul, Vatcharin,Pakawatchai, Chaveng,Saithong, Saowanit,Phongpaichit, Souwalak,Preedanon, Sita,Sakayaroj, Jariya
, p. 50 - 54 (2014)
A new phenolic glucoside, acremonoside (...
Catalytic properties and amino acid sequence of endo-1→3-β-D- glucanase from the marine mollusk Tapes literata
Zakharenko, A. M.,Kusaykin, M. I.,Kovalchuk, S. N.,Sova, V. V.,Silchenko, A. S.,Anastyuk, S. D.,Rasskazov, V. A.,Zvyagintseva, T. N.,Belik, A. A.,Ly, Bui Minh
, p. 878 - 888,11 (2012)
A specific 1→3-β-D-glucanase with molecu...
Two new secondary metabolites from xylaria sp. cfcc 87468
Wang, Fuqian,Han, Shishi,Hu, Song,Xue, Yongbo,Wang, Jianping,Xu, Hongfeng,Chen, Lu,Zhang, Geng,Zhang, Yonghui
, p. 1250 - 1257 (2014)
A new isocoumarin glycoside, 3R-(+)-5-O-...
Yihx-encoded haloacid dehalogenase-like phosphatase HAD4 from Escherichia coli is a specific α-d-glucose 1-phosphate hydrolase useful for substrate-selective sugar phosphate transformations
Pfeiffer, Martin,Wildberger, Patricia,Nidetzky, Bernd
, p. 39 - 46 (2014)
Phosphomonoester hydrolases (phosphatase...
Aglycon specificity profiling of α-glucosidases using synthetic probes
Hakamata, Wataru,Muroi, Makoto,Kadokura, Kazunari,Nishio, Toshiyuki,Oku, Tadatake,Kimura, Atsuo,Chiba, Seiya,Takatsuki, Akira
, p. 1489 - 1492 (2005)
We designed and synthesized hydrogen bon...
Protein nanotubes with an enzyme interior surface
Komatsu, Teruyuki,Terada, Hiromi,Kobayashi, Nao
, p. 1849 - 1854 (2011)
This report describes the synthesis and ...
Antibacterial activity of glucomoringin bioactivated with myrosinase against two important pathogens affecting the health of long-term patients in hospitals
Galuppo, Maria,De Nicola, Gina Rosalinda,Iori, Renato,Dell'Utri, Pia,Bramanti, Placido,Mazzon, Emanuela
, p. 14340 - 14348 (2013)
Glucosinolates (GLs) are natural compoun...
Cellodextrin phosphorylase from Ruminiclostridium thermocellum: X-ray crystal structure and substrate specificity analysis
O'Neill, Ellis C.,Pergolizzi, Giulia,Stevenson, Clare E.M.,Lawson, David M.,Nepogodiev, Sergey A.,Field, Robert A.
, p. 118 - 132 (2017)
The GH94 glycoside hydrolase cellodextri...
Purification, antioxidant activity and antiglycation of polysaccharides from Polygonum multiflorum Thunb
Lv, Lishuang,Cheng, Yunhui,Zheng, Tiesong,Li, Xiaoming,Zhai, Rong
, p. 765 - 773 (2014)
Polysaccharides, one of the most importa...
A novel macrolactam-disaccharide antifungal antibiotic taxonomy, fermentation, isolation, physico-chemical properties, structure elucidation and biological activity
Hegde,Patel,Gullo,Horan,King,Gentile,Wagman,Puar,Loebenberg
, p. 1109 - 1115 (1993)
A novel natural product (1), with antifu...
Asplenetin, a flavone and its glycoside from Launaea asplenifolia
Gupta,Ahmed, Bahar
, p. 873 - 875 (1985)
A new flavone, asplenetin, has been isol...
Hepatoprotective triterpenoid saponins from Callicarpa nudiflora
Huang, Bo,Fu, Hui-Zheng,Chen, Wei-Kang,Luo, Yue-Hua,Ma, Shuang-Cheng
, p. 695 - 699 (2014)
Four new triterpenoid saponins, 2α,3α,19...
Sonochemical synthesis of HSiW/graphene catalysts for enhanced biomass hydrolysis
Klein, Miri,Varvak, Alexander,Segal, Elad,Markovsky, Boris,Pulidindi, Indra Neel,Perkas, Nina,Gedanken, Aharon
, p. 2418 - 2425 (2015)
Hydrolysis of biomass for the production...
Bifunctional heterogeneous catalysts derived from the coordination of adenosine monophosphate to Sn(iv) for effective conversion of sucrose to 5-hydroxymethylfurfural
Ji, Peijun,Jiao, Lutong,Meng, Han,Wang, Chenyu
, p. 630 - 640 (2022/02/09)
Adenosine 5′-monophosphate (AMP) with mu...
Exopolysaccharide Produced by Probiotic Bacillus albus DM-15 Isolated From Ayurvedic Fermented Dasamoolarishta: Characterization, Antioxidant, and Anticancer Activities
Kalimuthu, Palanisamy,Ma, Yongkun,Mathivanan, Krishnamurthy,Rai, Amit Kumar,Saravanan, Kandasamy,Sathiyanarayanan, Ganesan,Sekar, Soundarapandian,Sudharsan, Kumaresan,Vinothkanna, Annadurai
, (2022/03/31)
An exopolysaccharide (EPS) was purified ...
Enzyme aggregation and fragmentation induced by catalysis relevant species
Adair, James,Bhide, Ashlesha,Gentile, Kayla,Ghosh, Subhadip,Kauffman, Joshua,Lee, Tae-Hee,Maiti, Subhabrata,Sen, Ayusman
, p. 20709 - 20717 (2021/10/02)
It is usually assumed that enzymes retai...
New benzoic acid and caffeoyl derivatives with anti-inflammatory activities isolated from leaves of Ilex kaushue
Kakumu, Yuya,Mitsunaga, Tohru,Nguyen, Thi Minh Tu,Yamauchi, Kosei
, (2021/08/16)
A new benzoic acid, 3-[2-(2-hydroxypheny...
492-62-6 Process route
-
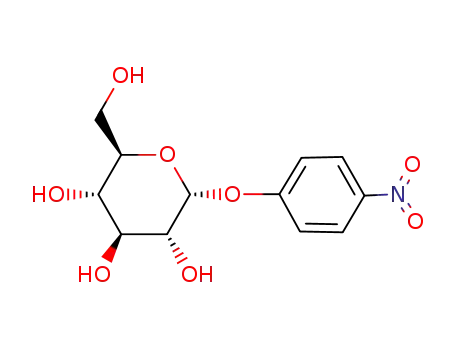
- 3767-28-0
4-nitrophenyl-α-D-glucopyranoside

-
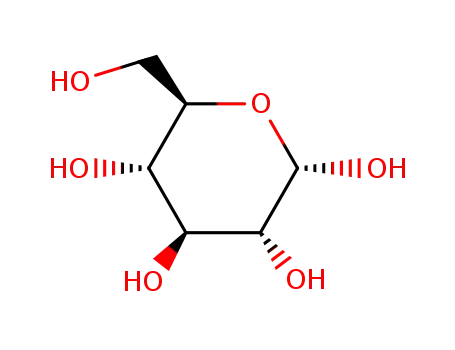
- 492-62-6
alpha-D-glucopyranose

-
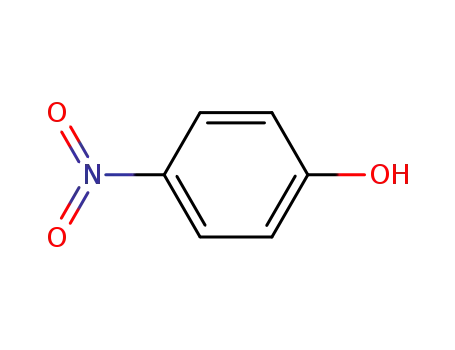
- 100-02-7,78813-13-5,89830-32-0
4-nitro-phenol
| Conditions | Yield |
|---|---|
|
With B. cereus oligo-1,6-glucosidase EC 3.2.1.10; In phosphate buffer; at 35 ℃; pH=7.0; Further Variations:; Reagents; Enzyme kinetics;
|
|
|
With baker's yeast α-glucosidase; water; acarbose; at 37 ℃; pH=7; Reagent/catalyst; Kinetics; aq. phosphate buffer; Enzymatic reaction;
|
|
|
With malA gene from Sulfolobus solfataricus; at 85 ℃; pH=4.5; Kinetics; aq. acetate buffer; Enzymatic reaction;
|
|
|
With α-glucosidase; prinsoside A; In aq. phosphate buffer; dimethyl sulfoxide; at 37 ℃; for 0.25h; pH=6.8; Reagent/catalyst; Kinetics; Enzymatic reaction;
|
|
|
With α-glycosidase; C40H59Cu2N12O5(1+); In methanol; acetonitrile; pH=10.5; pH-value; Kinetics; Enzymatic reaction;
|
|
|
With α-glucosidase on Caco-2 cells from colon carcinoma; In aq. phosphate buffer; at 37 ℃; for 1.5h; pH=7.4; Enzymatic reaction;
|
|
|
With Saccharomyces cerevisiae α-glycosidase; In aq. phosphate buffer; at 30 ℃; pH=7.4; Enzymatic reaction;
|
-
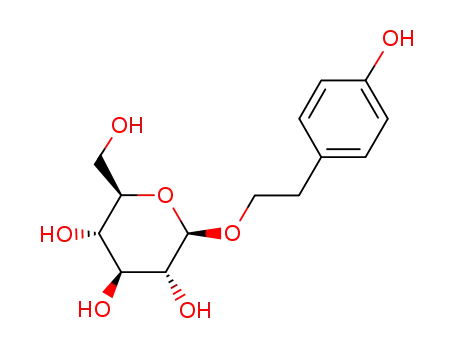
- 10338-51-9
Salidroside

-

- 492-62-6
alpha-D-glucopyranose
| Conditions | Yield |
|---|---|
|
With hydrogenchloride; water; In methanol; at 90 ℃; for 3h;
|
492-62-6 Upstream products
-
50-99-7
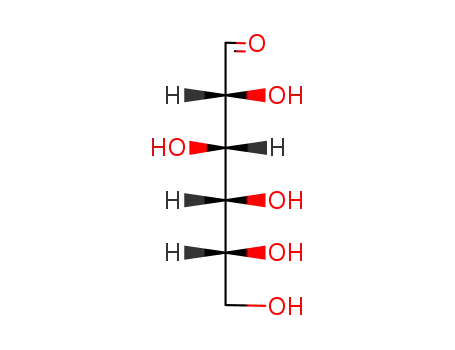
D-glucose
-
186581-53-3

diazomethane
-
67-56-1
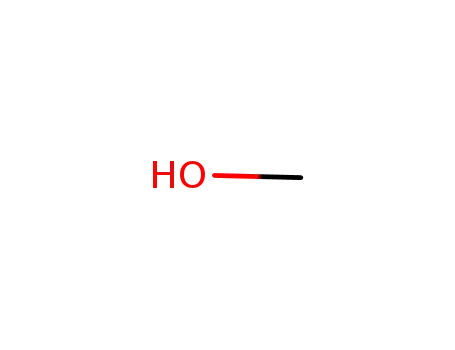
methanol
-
82203-02-9

nonacosa-O-methylagrimoniin
492-62-6 Downstream products
-
492-61-5
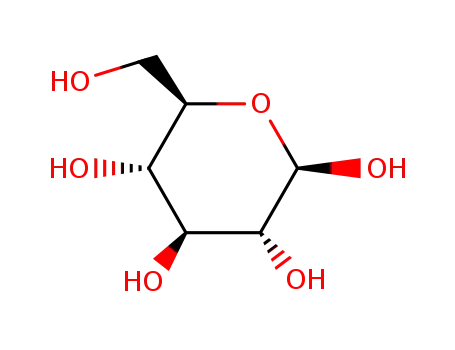
β-D-glucose
-
526-95-4
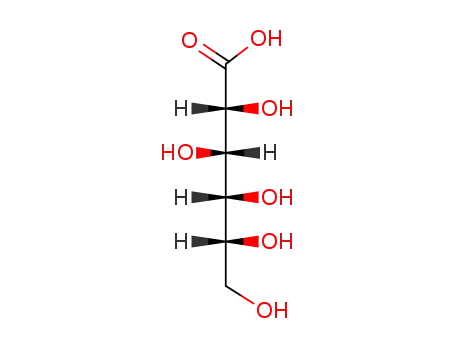
gluconic acid
-
22415-91-4
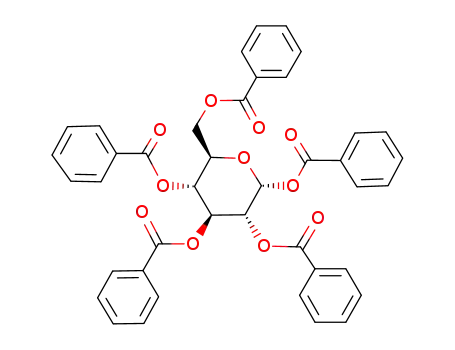
1,2,3,4,6-penta-O-benzoyl-α-D-glucopyranose
-
35385-01-4
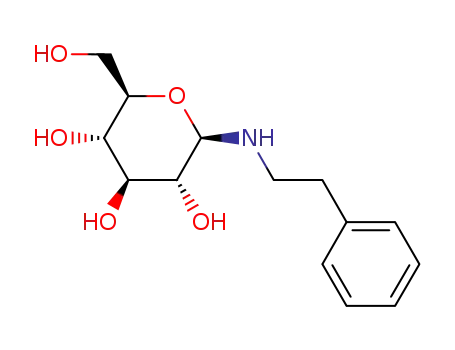
N-phenethyl-β-D-glucopyranosylamine
Relevant Products
-
Malic acid
CAS:6915-15-7
-
Sodium alginate
CAS:9005-38-3
-
Acesulfame
CAS:33665-90-6